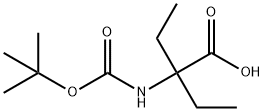
2-(tert-butoxycarbonylaMino)-2-ethylbutanoic acid synthesis
- Product Name:2-(tert-butoxycarbonylaMino)-2-ethylbutanoic acid
- CAS Number:139937-99-8
- Molecular formula:C11H21NO4
- Molecular Weight:231.29
![Carbamic acid, N-[1-ethyl-1-(hydroxymethyl)propyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1144505-60-1.gif)
1144505-60-1
0 suppliers
inquiry

139937-99-8
16 suppliers
inquiry
Yield:139937-99-8 73%
Reaction Conditions:
with ruthenium(IV) oxide;sodium periodate in tetrachloromethane;water;acetonitrile at 20; for 2 h;
Steps:
Preparation of N-Boc-Protected Amino Acids 7a-g from 4a-g;General Procedure
General procedure: To a solution of N-protected amino alcohol 4a-g (0.5 mmol) in a 2:2:3 mixture of CCl4/MeCN/H2O (7 mL) were successively added NaIO4(321 mg, 1.5 mmol) and RuO2·xH2O (3.5 mg, 0.025 mmol) at r.t. Theresulting dark mixture was vigorously stirred for 2 h at r.t. thenCH2Cl2 (10 mL) and H2O (10 mL) were added. The layers were separatedand the aqueous phase was extracted with CH2Cl2 (2 × 10 mL). Thecombined organic layers were washed with brine, dried over MgSO4,filtered and the solvent was removed in vacuo. The black residue waspurified by chromatography on silica gel (CH2Cl2-MeOH, 99:1 to95:5) to afford the N-Boc-protected amino acid 7a-g.
References:
Boukattaya, Fatma;Caillé, Julien;Ammar, Houcine;Rouzier, Florian;Boeda, Fabien;Pearson-Long, Morwenna S. M.;Bertus, Philippe [Synthesis,2016,vol. 48,# 6,art. no. 48,p. 906 - 916]
2566-29-2
82 suppliers
$50.00/250MG

24424-99-5
819 suppliers
$13.50/25G

139937-99-8
16 suppliers
inquiry

24424-99-5
819 suppliers
$13.50/25G

19792-52-0
12 suppliers
inquiry

139937-99-8
16 suppliers
inquiry