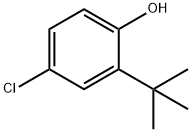
2-tert-Butyl-4-chlorophenol synthesis
- Product Name:2-tert-Butyl-4-chlorophenol
- CAS Number:13395-85-2
- Molecular formula:C10H13ClO
- Molecular Weight:184.66
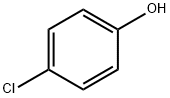
106-48-9
471 suppliers
$10.00/5 g
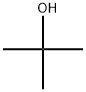
75-65-0
742 suppliers
$10.00/10ml

13395-85-2
55 suppliers
$30.00/25mg
Yield: 71%
Reaction Conditions:
with sulfuric acid for 48 h;Inert atmosphere;
Steps:
1 Salicylaldehyde Synthesis.
The ligand precursor 3-ter -butyl-5-chlorosalicylaldehyde has been previously reported, and FontWeight="Bold" FontSize="10" H NMR assignments are included that match well with those in the literature. The yield represents average isolated yields. The precursor to the salicylaldehyde, 2-tert- butyl-4-chlorophenol, was synthesized according to literature procedure. 4-Chlorophenol (Combi-B locks, 8.00 g, 62.2 mmol) was dissolved in tert-butyl alcohol (Aldrich, 1 1.9 mL, 124 mmol), and 7.50 mL of concentrated H2S04 was added dropwise over 5 minutes, turning the solution from a pale yellow to a light orange. The solution was stirred for 2 days, neutralized with Na2C03 (aq), and then extracted into diethyl ether and dried over Na2S04. The product was concentrated and purified by column chromatography (95:5, hex:EtOAc) resulting in a yellow oil (71% isolated yield). Using a modified Duff reaction, 2-/ert-butyl-4- chlorophenol was formylated as reported by Jacobsen et al. The product was purified by column chromatography (90: 10, hex:EtOAc) to yield a crystalline yellow solid (24% isolated yield). NMR spectrum in ppm (CDC13, 400 MHz): δ 1 1.72 (s, I H); 9.82 (s, IH); 7.46 (d, J = 2.6 Hz, IH); 7.38 (d, J = 2.6 Hz, IH); 1.41 (s, 12H).
References:
CORNELL UNIVERSITY;COATES, Geoffrey;WHITEHEAD, Julie WO2016/25675, 2016, A1 Location in patent:Paragraph 0100
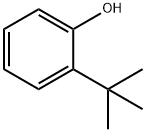
88-18-6
168 suppliers
$16.00/25mL

13395-85-2
55 suppliers
$30.00/25mg
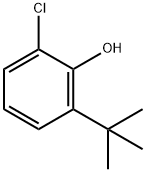
4237-37-0
19 suppliers
inquiry

88-18-6
168 suppliers
$16.00/25mL

13395-85-2
55 suppliers
$30.00/25mg

88-18-6
168 suppliers
$16.00/25mL

13395-85-2
55 suppliers
$30.00/25mg

4237-37-0
19 suppliers
inquiry
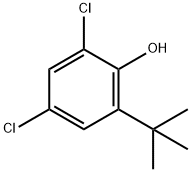
13395-86-3
1 suppliers
inquiry