2-(TERT-BUTYLDIMETHYLSILYL)-1,3-DITHIANE synthesis
- Product Name:2-(TERT-BUTYLDIMETHYLSILYL)-1,3-DITHIANE
- CAS Number:95452-06-5
- Molecular formula:C10H22S2Si
- Molecular Weight:234.5
505-23-7
201 suppliers
$16.00/1g

18162-48-6
665 suppliers
$9.00/5g

95452-06-5
14 suppliers
$75.50/1g
Yield:95452-06-5 95%
Reaction Conditions:
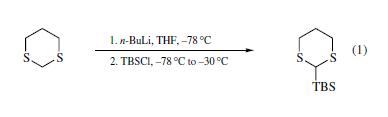
Stage #1: dithianewith n-butyllithium in tetrahydrofuran;hexane at -30; for 1 h;
Stage #2: tert-butyldimethylsilyl chloride in tetrahydrofuran;hexane at -78 - 20; for 16 h;
Steps:
tert-Butyl(1,3-dithian-2-yl)dimethylsilane (1a)
n-BuLi (20.0 mmol, 12.5 mL, 1.20 equiv, 1.60 M in n-hexane) was added dropwise to a stirred solution of 1,3-dithiane (2.00 g, 16.6 mmol) in tetrahydrofuran (33 mL) at -30°C. The reaction mixture was stirred for 1 h, then cooled to -78°C, tert-butyldimethylsilyl chloride (3.01 g, 20.0 mmol 1.20 equiv) in tetrahydrofuran (10 mL) was added, and the mixture was gradually warmed to room temperature and stirred for another 16 h. Saturated aqueous NH4Cl solution (50 mL) was added. Diethyl ether (50 mL) was used to dilute the reaction mixture, which was then washed with H2O (50 mL × 2) and sat. aqueous NaCl (50 mL× 2). The organic layer was dried over MgSO4, and filtered. The filtrate was concentrated under reduced pressure. The crude product was purified by column chromatography (silica gel, n-hexane/EtOAc = 98:2) to give 1a (3.71 g, 95%) as a colorless oil: Rf = 0.36 (silica gel, n-hexane/EtOAc = 98:2); 1H-NMR (400 MHz, CDCl3) δ 3.81 (s, 1H), 2.94-2.86 (m, 2H), 2.71 (dt, J = 13.9, 3.7 Hz, 2H), 2.14-1.95 (m, 2H), 0.98 (s, 9H), 0.12 (s, 6H); 13C-NMR (101 MHz, CDCl3) δ 32.5, 31.5 (2C), 27.0 (3C), 26.2, 17.6, -7.2 (2C); IR (neat) 2953, 2929, 2897, 2856, 1470, 1421, 1250, 909, 879, 826, 810, 775 cm-1; EI-MS (70 eV) m/z (relative intensity): 234 (50, M+) 177 (100, M - C4H9), 149 (72), 119 (23), 91 (14), 87 (77), 73 (92), 59 (19); HRMS (EI) m/z: calcd for C10H22S2Si: 234.0932, found 234.0934; Anal. calcd for C10H22S2Si: C, 51.22; H, 9.46; N, 0.00, found: C, 51.40; H, 9.73; N, 0.00. 1H NMR data were consistent with the reported data.1
References:
Noji, Masahiro;Ishimaru, Sho;Obata, Haruki;Kumaki, Ayano;Seki, Taichi;Hayashi, Satoshi;Takanami, Toshikatsu [Tetrahedron Letters,2022,vol. 104,art. no. 154026] Location in patent:supporting information