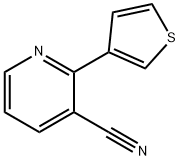
2-(Thiophen-3-yl)nicotinonitrile synthesis
- Product Name:2-(Thiophen-3-yl)nicotinonitrile
- CAS Number:870065-06-8
- Molecular formula:C10H6N2S
- Molecular Weight:186.23
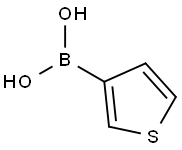
6165-69-1
341 suppliers
$12.72/1gm:
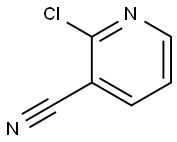
6602-54-6
494 suppliers
$6.00/10g

870065-06-8
15 suppliers
inquiry
Yield:870065-06-8 56%
Reaction Conditions:
with potassium fluoride;tris-(dibenzylideneacetone)dipalladium(0) in 1,4-dioxane at 90; for 18 h;
Steps:
96.A 2-thiophen-3-ylnicotinonitrile
EXAMPLE 96A 2-thiophen-3-ylnicotinonitrile To an oven-dried, N2-purged, 50 mL, round-bottomed flask containing a magnetic stir bar were added potassium fluoride (767 mg, 13.2 mmol), bis(tri-t-butylphosphine)palladium (51.0 mg, 0.10 mmol), tris(dibenzylideneacetone)dipalladium (46 mg, 0.05 mmol), 2-chloro-3-cyanopyridine (559 mg, 4.00 mmol), and 3-thiopheneboronic acid (819 mg, 6.4 mmol). The flask was sealed with a septum and purged with dry N2 atmosphere. Anhydrous dioxane (4 mL) was added via syringe. The reaction mixture was heated to ~90° C. in an oil bath for 18 hours. After cooling to room temperature, ethyl acetate (15 mL) was added and the mixture was filtered through a pad of silica. The filtrate was concentrated by rotary evaporation to give a brown oil. The product was purified by recrystallization from ethyl acetate/hexanes to give 417 mg (56%) of the title compound. MS (ESI-) m/z 186.9 (M+H)+; 1H NMR (CDCl3) δ 7.30 (7.8, 4.7 Hz, 1H), 7.44 (dd, J=5.3, 2.9 Hz, 1H), 7.88 (dd, J=5.1, 1.4 Hz, 1H), 8.03 (dd, J=8.0, 1.9 Hz, 1H), 8.29 (dd, J=3.0, 1.4 Hz, 1H), 8.82 (dd, J=4.7, 1.7 Hz, 1H).
References:
US2007/105842,2007,A1 Location in patent:Page/Page column 28-29

21298-55-5
53 suppliers
$19.00/100mg

870065-06-8
15 suppliers
inquiry
937601-80-4
14 suppliers
inquiry

21298-55-5
53 suppliers
$19.00/100mg

870065-06-8
15 suppliers
inquiry