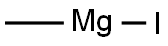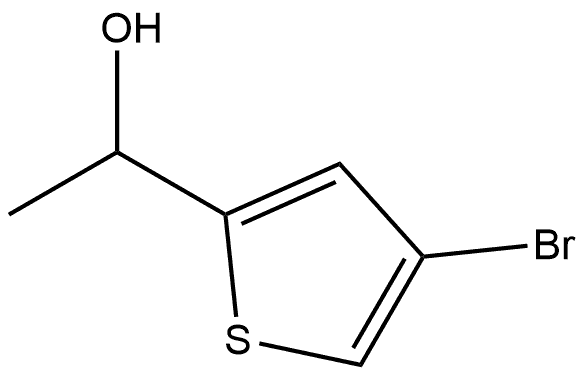
2-THIOPHENEMETHANOL, 4-BROMO-ALPHA-METHYL- synthesis
- Product Name:2-THIOPHENEMETHANOL, 4-BROMO-ALPHA-METHYL-
- CAS Number:34878-46-1
- Molecular formula:C6H7BrOS
- Molecular Weight:207.09

18791-75-8
275 suppliers
$9.00/5g

34878-46-1
6 suppliers
inquiry
Yield:34878-46-1 98.5%
Reaction Conditions:
with ammonium chloride;methyllithium in diethyl ether;water;
Steps:
20.a a)
a) Preparation of 4-bromo-2-(1'-hydroxyethyl)thiophene A solution of 4-bromo-2-thiophenecarboxaldehyde (Aldrich, 1.20 g, 6.28 mmol) in 15 ml of anhydrous ethyl ether is brought to -70° C. in a flask fitted with a thermometer, under an atmosphere of argon and with magnetic stirring. Then a 1.6 M solution of MeLi in ether (4.12 ml, 6.59 mmol) is added drop by drop between -70° C. and -60° C. The reaction is followed by CCM (eluent ether:petroleum ether=20:80). The mixture is hydrolyzed in the cold with 5 ml of saturated NH4Cl solution, 20 ml of distilled water is added and the mixture is extracted with ether (3*40 ml). The ether phase is dried over MgSO4, filtered and evaporated to obtain 1.28 g of a slightly yellowish oil, 4-bromo-2-(1'-hydroxyethyl)thiophene (gross yield=98.5%). NMR1H 200 MHz (CDCl3): 1.53 (d, 3H, Me J 6 Hz); 2.3-2.5 (s broad, 1H mobile, -OH); 5.02 (q, 1H, -CHOH J 6.0 Hz); 6.84 (d, 1H, ArH J 1.5 Hz); 7.09 (d, 1H, ArH J 1.5 Hz).
References:
US6265423,2001,B1
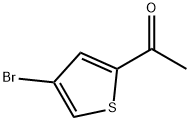
7209-11-2
108 suppliers
$16.00/250mg

34878-46-1
6 suppliers
inquiry
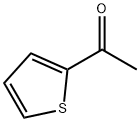
88-15-3
599 suppliers
$5.00/10g

34878-46-1
6 suppliers
inquiry