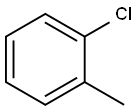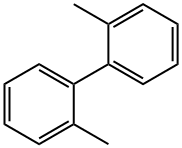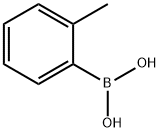
2-Tolylboronic acid synthesis
- Product Name:2-Tolylboronic acid
- CAS Number:16419-60-6
- Molecular formula:C7H9BO2
- Molecular Weight:135.96
Yield:16419-60-6 97%
Reaction Conditions:
with cesium fluoride;Pd(dba)2 in toluene;
Steps:
5 Example 5
Example 5 This is an example of Pd/ligand 1-catalyzed Suzuki reaction for biaryl synthesis 2,2'-Dimethyl-1,1'-biphenyl The title compound was obtained as a yellowish oil (180 mg, 97% isolated yield) from the reaction of 2-methyl-phenylboronic acid (203 mg, 1.54 mmol), CsF (469 mg, 3.09 mmol), Pd(dba)2 (11.8 mg, 21 μmol), ligand 1 (22 mg, 61 μmol), and 2-chlorotoluene (0.12 mL, 1.03 mmol) in toluene (4 mL) at 110° C. for 5 h. TON=49 and TOF=9.8. 1 H NMR (CDCl3): δ7.32-7.18 (m, 6H, ArH), 7.13 (d, J=6.2, 2H, ArH), 2.08 (s, 6H, CH3's). 13C{1H} NMR (CDCl3): δ141.6, 135.8, 129.8, 129.3, 127.1, 125.5, 19.8.
References:
US6268513,2001,B1
95-46-5
291 suppliers
$10.00/10g

16419-60-6
370 suppliers
$9.00/1g

66107-34-4
38 suppliers
$30.00/1g

16419-60-6
370 suppliers
$9.00/1g
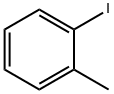
615-37-2
286 suppliers
$5.00/5g

16419-60-6
370 suppliers
$9.00/1g
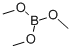
121-43-7
346 suppliers
$14.00/25mL

5419-55-6
371 suppliers
$12.00/5g

95-46-5
291 suppliers
$10.00/10g

16419-60-6
370 suppliers
$9.00/1g