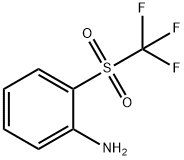
2-trifluoromethanesulfonylaniline synthesis
- Product Name:2-trifluoromethanesulfonylaniline
- CAS Number:17095-18-0
- Molecular formula:C7H6F3NO2S
- Molecular Weight:225.19
Yield:17095-18-0 87%
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 0.166667 h;Inert atmosphere;Reagent/catalyst;Solvent;
Steps:
1; 1; 2 Example 1, 2-((trifluoromethyl)sulfonyl)aniline
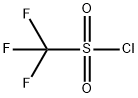
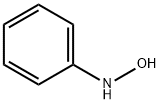
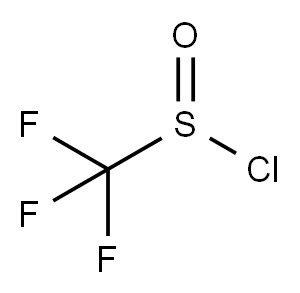
Under nitrogen atmosphere, N-phenylhydroxylamine (0.2 mmol, 1.0 eq.) was dissolved in an ultra-dry solution of dichloromethane (2 mL),and cooled to 0°C. Then triethylamine (0.3 mmol, 1.5 eq.) was added slowly, and trifluoromethanesulfonyl chloride (0.24 mmol, 1.2 eq.) was added dropwise with stirring. The reaction mixture was stirred at 0°C for 10 minutes. After the reaction was completed, the reaction mixture was diluted with DCM and the solvent was removed by rotary evaporation. The crude product was subjected to column chromatography (eluent: petroleum ether:ethyl acetate=10:1) After purification, pure 2-((trifluoromethyl)sulfonyl)aniline was obtained as a colorless oil with a yield of 87%.
References:
CN113929605,2022,A Location in patent:Paragraph 0078-0083; 0131-0141
![1H-Isoindole-1,3(2H)-dione, 2-[2-[(trifluoromethyl)sulfonyl]phenyl]-](/CAS/20210305/GIF/22351-78-6.gif)
22351-78-6
0 suppliers
inquiry

17095-18-0
11 suppliers
inquiry

384-37-2
3 suppliers
inquiry

17095-18-0
11 suppliers
inquiry
3686-57-5
24 suppliers
$45.00/100mg

17095-18-0
11 suppliers
inquiry