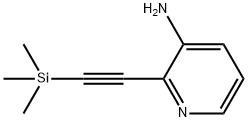
2-((Trimethylsilyl)ethynyl)pyridin-3-amine synthesis
- Product Name:2-((Trimethylsilyl)ethynyl)pyridin-3-amine
- CAS Number:947330-64-5
- Molecular formula:C10H14N2Si
- Molecular Weight:190.32

39856-58-1
309 suppliers
$10.00/1g
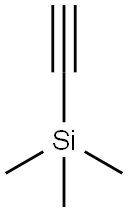
1066-54-2
470 suppliers
$9.00/10g

947330-64-5
27 suppliers
$155.00/50mg
Yield: 80%
Reaction Conditions:
with copper(l) iodide;tetrakis(triphenylphosphine) palladium(0);triethylamine in tetrahydrofuran at 60; for 6 h;Inert atmosphere;Sonogashira Cross-Coupling;
Steps:
8.1 3.1 Example C
General procedure: The catalyst [Pd(PPh3)4] (5 mol %) was added to a degassed solution of iodine azaindole derivative (1.0 eq.), alkyne (1.2 eq.) and CuI (10 mol %) in a mixture of Et3N/THF (1:1) at a concentration of 0.1 M. The mixture was heated at 50-60° C. for 4 h under argon atmosphere. After returning to room temperature, the mixture was poured into an aqueous solution of (10%) NH4Cl. After phase separation, the aqueous phase was further extracted with ethyl acetate (3 times 20 ml) and the combined organic phases were washed with NaCl, dried over MgSO4, filtered, concentrated under reduced pressure and purified by flash column chromatography on silicagel
References:
CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE;UNIVERSITE D' ORLEANS;UNIVERSITE FRANCOIS RABELAIS DE TOURS;CENTRE HOSPITALIER REGIONAL UNIVERSITAIRE DE TOURS;INSERM (INSTITUT NATIONAL DE LA SANTE ET DE LA RECHERCHE MEDICALE;ROUTIER, Sylvain;SUZENET, Franck;CHALON, Sylvie;BURON, Frederic;VERCOUILLIE, Johnny;MELKI, Ronald;BOIARYNA, Liliana;GUILLOTEAU, Denis;PIERI, Laura Ronald US2019/211011, 2019, A1 Location in patent:Paragraph 0576; 0587; 0588
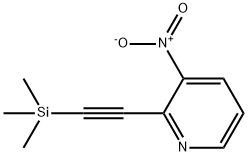
107469-26-1
4 suppliers
inquiry

947330-64-5
27 suppliers
$155.00/50mg
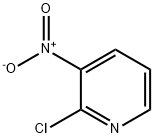
5470-18-8
439 suppliers
$6.00/5g

947330-64-5
27 suppliers
$155.00/50mg

1066-54-2
470 suppliers
$9.00/10g

947330-64-5
27 suppliers
$155.00/50mg
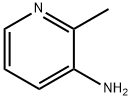
3430-10-2
248 suppliers
$7.00/1g

1066-54-2
470 suppliers
$9.00/10g

947330-64-5
27 suppliers
$155.00/50mg