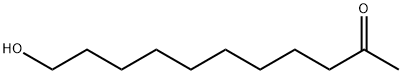
2-Undecanone, 11-hydroxy- synthesis
- Product Name:2-Undecanone, 11-hydroxy-
- CAS Number:35345-72-3
- Molecular formula:C11H22O2
- Molecular Weight:186.29

112-43-6
266 suppliers
$5.00/1g

35345-72-3
1 suppliers
inquiry
Yield:35345-72-3 98%
Reaction Conditions:
with tert.-butylhydroperoxide;silver(I) hexafluorophosphate;[Pd(Quinox)Cl2] in dichloromethane;water at 0 - 20; for 0.583333 h;Product distribution / selectivity;Protection from light;Tsuji-Wacker oxidation;
Steps:
General TBHP Mediated Wacker Reaction:Table 1 reports individual examples while the following description provides a common reaction scheme used in each example with deviations noted. In the dark, AgSbF6 (51.5 mg, 0.15 mmol), Pd(quinox)Cl2 complex (22.5 mg, 0.06 mmol), and a magnetic stir bar were added to a 100 mL round bottomed flask. DCM (4.8 mL) was added to the flask and the mixture was stirred for 15 min. The mixture was then diluted with DCM (20 mL) and 70 wt % TBHP(aq) (5.2 mL, 36 mmol) was added. The resulting mixture was stirred for an additional 10 min, before being cooled in an ice bath. Once the solution had cooled, the substrate (3.0 mmol) was added with stirring. After 5 min, the ice bath was removed and the reaction mixture was allowed to slowly warm to room temperature. Once TLC indicated complete consumption of starting material, the reaction was quenched with a saturated aqueous solution of Na2SO3 (50 mL) to consume excess TBHP. The mixture was transferred to a separatory funnel and diluted with hexanes (50 mL). The aqueous layer was separated and back extracted with hexanes (25 mL). The combined organics were washed with water (4×25 mL) and brine (50 mL). The combined organic phases were dried over MgSO4, filtered and concentrated under reduced pressure. The crude material was purified by silica gel flash chromatography if necessary; the product containing fractions were combined and concentrated under reduced pressure. The general reaction scheme was as follows:; Several common protecting groups for allylic alcohols were shown to be compatible with these conditions, providing the methyl ketone products in high yields (Table 1, entries 1-4). No aldehyde product was detected in any of these cases by GC or 1H NMR analysis. Thus, the above approach can be substantially free or completely free of undesired aldehyde products. Additionally, silyl protected allylic alcohols proceed to product at a significantly faster rate, allowing for the use of lower catalyst loadings (entry 2). An acetal-protecting group, was stable to Lewis acidic, aqueous reaction conditions (entry 3). A homoallylic alcohol also was converted to the methyl ketone in excellent yield (entry 5).The corresponding ketones were each confirmed using NMR, IR and HRMS analyses. The 2-oxooctan-3-yl acetate, 3-(tert-butyldimethylsiloxy)octan-2-one, 3-(ethoxymethoxy)octan-2-one, 1-cyclohexyl-2-oxopropyl acetate, 4-(tert-butyldimethylsolxy)-4-phenylbutan-2-one, 2-decanone, methyl 10-oxoundecanoate, 6-(2,2-dimethyl-1,3-dioxolan-4-yl)hexan-2-one, 11-chloroundecan-2-one, 1-p-tolyethanone were recovered as colorless oils. The 11-hydroxyundecan-2-one, tert-butyl 4-acetylphenylcarbamate, and 1-(3-nitro)ethanone were recovered as a white solid having a melting point of 39-40° C., 113-114° C., and 68-70° C., respectively. Similarly, 11-hydroxyundecan-2-one was isolated as a white solid.The catalytic system was further evaluated to determine whether it was a general catalyst for the Wacker oxidation. Excitingly, the current system rapidly consumes decene leading to 2-decanone in good yield with no appreciable amounts of internal isomers (entry 6). Lowering the amount of TBHP led to conversion of decene without significant loss of yield or increase in isomerization (entry 7). Other functional groups were evaluated including an alkene with a free distal alcohol, which was used to demonstrate the scalability of the reaction using a reduced catalyst loading (entries 8 and 9). Additionally, alkenes containing common functional groups including a methyl ester, acetonide, and primary chloride are well tolerated (entries 10-12). A number of styrene derivatives, another challenging substrate class for the Tsuji-Wacker oxidation, were also evaluated. Substituted styrenes with various functional groups were efficiently oxidized, although a highly electron-deficient system led to a lower yield and a trace amount of m-nitro benzaldehyde (entries 13-15). It should be noted that these reactions are exothermic; therefore the oxidations were initiated at 0° C.
References:
US2011/54176,2011,A1 Location in patent:Page/Page column 9-10

2774-84-7
113 suppliers
$45.00/1g

35345-72-3
1 suppliers
inquiry
10596-05-1
0 suppliers
inquiry

35345-72-3
1 suppliers
inquiry
38199-58-5
0 suppliers
inquiry

35345-72-3
1 suppliers
inquiry
36219-78-0
0 suppliers
inquiry

35345-72-3
1 suppliers
inquiry