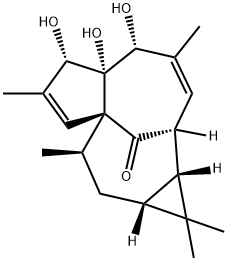
20-DEOXYINGENOL synthesis
- Product Name:20-DEOXYINGENOL
- CAS Number:54706-99-9
- Molecular formula:C20H28O4
- Molecular Weight:332.43
Yield: 28%
Reaction Conditions:
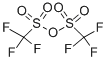
Stage #1:trifluoromethylsulfonic anhydride;(3S,3aS,6aR,7S,8R,9R,9aR,10aR,12R,12aS)-8-hydroxy-2,7,10,10,12pentamethyl-5,13-dioxo-6a,7,9,9a,10,10a,11,12-octahydro-3H,8H-9,12a-methanocyclopenta[1,10]cyclopropa[6,7]cyclodeca[1,2-d][1,3 ]dioxol-3-yl acetate with pyridine;dmap at 80; for 0.5 h;
Stage #2: with 1,8-diazabicyclo[5.4.0]undec-7-ene at 110; for 0.5 h;
Stage #3: with sodium hydroxide in water for 0.5 h;
Steps:
27
To a solution of 17 (9.0 mg, 0.0215 mmol, 1.0 equiv) and DMAP (0.26 mg, 0.00215 mmol, 0.1 equiv) in of pyridine (1 mL), was added Tf20 (11 μ, 0.0645 mmol, 3.0 equiv). The solution was warmed to 80 °C for 30 min. To the solution was added DBU (19 μ, 0.129 mmol, 6.0 equiv). The solution was heated to 110 °C for 30 min. The mixture was cooled and 10% aqueous sodium hydroxide (200 μ^) was added. The mixture was stirred for 30 min then quenched by the addition of 3 mL of saturated aqueous NaHC03 and 3 mL of EtOAc. The aqueous layer was removed and the organic layer was washed with saturated aqueous CuS04 (2 x 3 mL). The organic layer was dried and evaporated to give crude 20. The crude material was purified by column chromatography (50: 1 DCM/MeOH) to give 20-deoxyingenol (20, 2.0 mg, 28%).
References:
LEO LABORATORIES LIMITED;BARAN, Phillipe S.;JØRGENSEN, Lars;KUTTRUFF, Christian A.;MCKERRALL, Steven J.;YEH, Chien-Hung WO2014/191457, 2014, A1 Location in patent:Page/Page column 89; 90
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

54706-99-9
108 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

54706-99-9
108 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

54706-99-9
108 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

54706-99-9
108 suppliers
inquiry