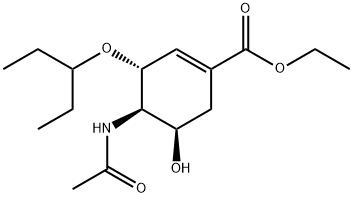
ETHYL (3R,4R,5S)-4-ACETAMIDO-5-AZIDO-3-(1-ETHYLPROPOXY)CYCLOHEX-1-ENE-1-CARBOXYLATE synthesis
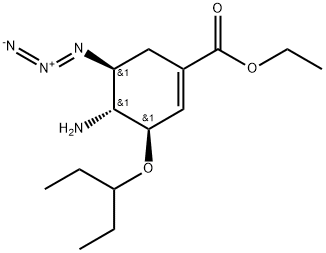
- Product Name:ETHYL (3R,4R,5S)-4-ACETAMIDO-5-AZIDO-3-(1-ETHYLPROPOXY)CYCLOHEX-1-ENE-1-CARBOXYLATE
- CAS Number:204255-06-1
- Molecular formula:C16H27N4O4
- Molecular Weight:0
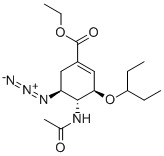
196618-13-0
213 suppliers
inquiry

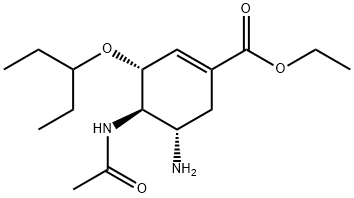
204255-06-1
89 suppliers
inquiry
Yield:204255-06-1 99%
Reaction Conditions:
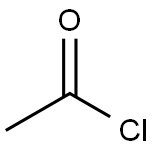
with N-diazosulfamoyl fluoride;potassium hydrogencarbonate in tert-butyl methyl ether;water;N,N-dimethyl-formamide at 20; for 0.5 h;
Steps:
General procedure for the preparation of the isolated azide compounds.
General procedure: To a 50-ml glass round-bottom flask was added sequentially the primary amine 2 (1.0 mmol; aliphatic, aromatic or heteroaromatic; as free naked amine or as HCl, MsOH, TsOH or tartrate salt), FSO2N3 solution (containing 1.0 mmol FSO2N3, approximately 200 mM in DMF/MTBE 1:1, v/v, approximately 5 ml, volume adjusted according to the concentration; prepared according to the above procedure and diluted with equal volume of DMF) and aqueous KHCO3 solution (3.0 M, 1.33 ml, containing 4.0 mmol KHCO3). The reaction mixture was stirred for 5 min at room temperature, while monitoring by LC-MS. After completion, EtOAc (40 ml) was added and the mixture was washed sequentially with brine (60 ml × 6), water (60 ml × 2) and brine (60 ml), dried over Na2SO4, concentrated by rotary evaporation and dried under vacuum to afford the azide product 3. For products containing acidic functional groups, this extraction process was modified with acidified aqueous phase (acidified with aqueous HCl). Detailed procedures and the various modifications, as well as the characterization data for each compound, can be found in Supplementary Information 1.
References:
Meng, Genyi;Guo, Taijie;Ma, Tiancheng;Zhang, Jiong;Shen, Yucheng;Sharpless, Karl Barry;Dong, Jiajia [Nature,2019,vol. 574,# 7776,p. 86 - 89]
1149345-89-0
0 suppliers
inquiry

204255-06-1
89 suppliers
inquiry
![7-Azabicyclo[4.1.0]hept-3-ene-3-carboxylic acid, 7-acetyl-5-(1-ethylpropoxy)-, ethyl ester, (1R,5R,6R)-](/CAS/20200401/GIF/1268086-96-9.gif)
1268086-96-9
2 suppliers
inquiry

204255-06-1
89 suppliers
inquiry
![1-Cyclohexene-1-carboxylic acid, 4-(acetylamino)-3-(1-ethylpropoxy)-5-[[(4-methylphenyl)sulfonyl]oxy]-, ethyl ester, (3R,4S,5R)-](/CAS/20210305/GIF/1001085-29-5.gif)
1001085-29-5
0 suppliers
inquiry

204255-06-1
89 suppliers
inquiry