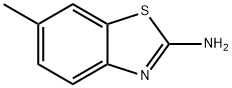
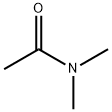
Acetamide, N-(6-methyl-2-benzothiazolyl)- (8CI,9CI) synthesis
- Product Name:Acetamide, N-(6-methyl-2-benzothiazolyl)- (8CI,9CI)
- CAS Number:20600-51-5
- Molecular formula:C10H10N2OS
- Molecular Weight:206.26
Yield:20600-51-5 72%
Reaction Conditions:
with Imidazole hydrochloride at 150; for 5 h;Sealed tube;
Steps:
3.2. General Procedures for the Synthesis of N-Acetamides 1a-1w
General procedure: To a mixture of aromatic or aliphatic or heterocyclic amine (3.0 mmol, 1.0 equiv.), Imidazolium chloride (1.0 mmol, 0.3 equiv.), N,N-Dimethyl acetamide (2.0 mL) was added. The mixture was refluxed at 150 °C and the progress of the reaction was monitored by TLC visualized with UV short wavelength followed by iodine stain. After completion, the mixture was diluted with cold water(10 mL) then extracted with EtOAc (10 mL). The EtOAc layer was washed with 1 M hydrochloric acid (3.0 15 mL). Adsorption of pigment with activated carbon, filtration of filtrate. The filtrate was dried over anhydrous Na2SO4 and concentrated under vacuum to obtain crude N-acetamide amine, the N-acetamide amine product was isolated by column chromatography eluting with petroleum ether:ethyl acetate (10:1) mixtures.
References:
Tian, Qingqiang;Gan, Zongjie;Wang, Xuetong;Li, Dan;Luo, Wen;Wang, Huajun;Dai, Zeshu;Yuan, Jianyong [Molecules,2018,vol. 23,# 9,art. no. 2234] Location in patent:supporting information
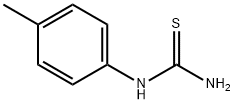
622-52-6
126 suppliers
$9.00/1g

20600-51-5
3 suppliers
inquiry
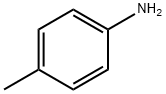
106-49-0
398 suppliers
$5.00/5G

20600-51-5
3 suppliers
inquiry