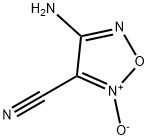
1,2,5-Oxadiazole-3-carbonitrile,4-amino-,2-oxide(9CI) synthesis
- Product Name:1,2,5-Oxadiazole-3-carbonitrile,4-amino-,2-oxide(9CI)
- CAS Number:206363-17-9
- Molecular formula:C3H2N4O2
- Molecular Weight:126.07
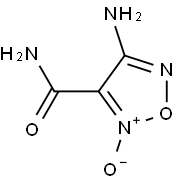
82295-76-9
4 suppliers
inquiry

206363-17-9
8 suppliers
inquiry
Yield:206363-17-9 84%
Reaction Conditions:
with pyridine;trifluoroacetic anhydride in acetonitrile at 0 - 20;
Steps:
1 4.3. General procedure for the synthesis of cyanofuroxans 1a-k
General procedure: To a magnetically stirred mixture of the appropriate furoxancarboxylic acid amide 3a-k (10 mmol) in dry CH2Cl2 (25 mL) or dry MeCN (20 mL) (see Table 1 for details) was added dry pyridine (1.61 mL, 20 mmol in case of amides 3a,c,d,f,h-k and 3.22 mL, 40 mmol in case of amides 3b,e,g) at room temperature. The resulting mixture was cooled to 0 °C in an ice-water bath and (CF3CO)2O (2.64 mL, 19 mmol in case of amides 3a,c,d,f,h-k and 5.28 mL, 38 mmol in case of amides 3b,e,g) was added dropwise at 0-5 °C. Then, the cooling bath was removed and the reaction mixture was stirred at room temperature for the appropriate period of time (see Table 1). Water (20 mL) was added. Cyanofuroxans 1a,b,f,i were extracted with CCl4 (3×10 mL), combined organic extracts were washed with water, then with 10% HCl and again with water and dried over MgSO4. In case of cyanofuroxans 1c,d,h after the addition of water the organic layer was separated, aqueous layer was extracted with CH2Cl2 (2×10 mL), combined organic extracts were washed with water, then with 10% HCl and again with water and dried over MgSO4. In case of cyanofuroxans 1e,g,j,k after the addition of water CH2Cl2 was evaporated under reduced pressure and the solid was filtered off, washed with water, and dried in air.
References:
Fershtat, Leonid L.;Epishina, Margarita A.;Kulikov, Alexander S.;Ovchinnikov, Igor V.;Ananyev, Ivan V.;Makhova, Nina N. [Tetrahedron,2015,vol. 71,# 38,p. 6764 - 6775]