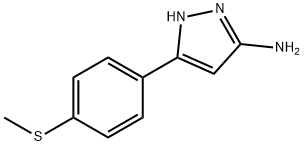
JR-13707, 3-(4-(Methylthio)phenyl)-1H-pyrazol-5-amine, 97% synthesis
- Product Name:JR-13707, 3-(4-(Methylthio)phenyl)-1H-pyrazol-5-amine, 97%
- CAS Number:208519-14-6
- Molecular formula:C10H11N3S
- Molecular Weight:205.28
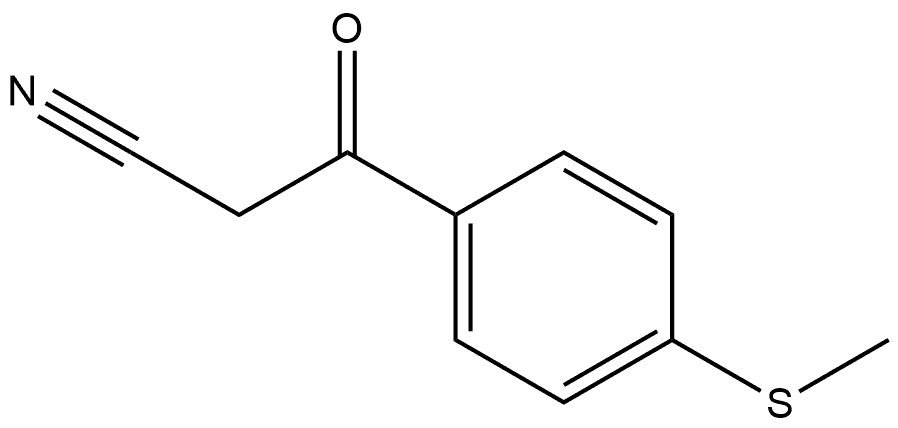
1344045-96-0
4 suppliers
inquiry

208519-14-6
4 suppliers
inquiry
Yield:208519-14-6 77%
Reaction Conditions:
with hydrogenchloride;hydrazine hydrate in ethanol;water; for 2.5 h;Reflux;
Steps:
2 5.1.2 3-[4-(Methylsulfanyl)phenyl]-1H-pyrazol-5-amine (5)
To a solution of 4 (2.0 g) in EtOH (10 mL) and H2O (10 mL) were added hydrazine hydrate (0.66 mL) and concentrated HCl (0.2 mL), and the reaction mixture was refluxed for 2.5 h. After cooling to room temperature, the mixture was poured into AcOEt/H2O. The mixture was made basic with saturated aqueous NaHCO3 and extracted. The organic layer was dried over anhydrous MgSO4 and concentrated in vacuo. The resulting solid was washed with EtOH/Et2O to give 5 (1.7 g, 77%) as a pale brown solid. Mp 164-165 °C; 1H NMR (DMSO-d6) δ 2.48 (3H, s), 5.73 (1H, br s), 7.25 (2H, d, J = 8.5 Hz), 7.58 (2H, d, J = 8.5 Hz); FAB MS m/e (M+H)+ 206.
References:
Inoue, Takayuki;Morita, Masataka;Tojo, Takashi;Nagashima, Akira;Moritomo, Ayako;Imai, Keisuke;Miyake, Hiroshi [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 9,p. 2478 - 2494]
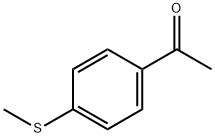
1778-09-2
216 suppliers
$10.00/1g

208519-14-6
4 suppliers
inquiry

42445-46-5
39 suppliers
$62.50/250mg

208519-14-6
4 suppliers
inquiry