
2-(2-CHLORO-ACETYLAMINO)-4,5,6,7-TETRAHYDRO-BENZO[B]THIOPHENE-3-CARBOXYLIC ACID AMIDE synthesis
- Product Name:2-(2-CHLORO-ACETYLAMINO)-4,5,6,7-TETRAHYDRO-BENZO[B]THIOPHENE-3-CARBOXYLIC ACID AMIDE
- CAS Number:20886-87-7
- Molecular formula:C11H13ClN2O2S
- Molecular Weight:272.75

79-04-9
352 suppliers
$12.00/5g
4815-28-5
157 suppliers
$10.00/500mg
![2-(2-CHLORO-ACETYLAMINO)-4,5,6,7-TETRAHYDRO-BENZO[B]THIOPHENE-3-CARBOXYLIC ACID AMIDE](/CAS/GIF/20886-87-7.gif)
20886-87-7
21 suppliers
$28.60/10MG
Yield:20886-87-7 84%
Reaction Conditions:
with triethylamine at 0 - 20; for 2 h;
Steps:
10 Example 10: r4-('Cvclopropyl-methyl-amino -5,6,7,8-tetrahvdro-benzor4,51thienor2,3- dlpyrimidin-2-vH -methanol (Compound 49)
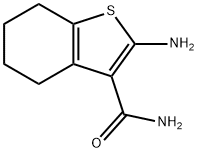
Example 10: r4-('Cvclopropyl-methyl-amino -5,6,7,8-tetrahvdro-benzor4,51thienor2,3- dlpyrimidin-2-vH -methanol (Compound 49) To a mixture of 2-amino-4,5,6,7-tetrahydro-l-benzothiophene-3-carboxamide (30 g, 0.153 mol) and triethylamine (18.5g, 0.183mol) in DCM (200 ml) was added chloro-acetyl chloride (20.57 g, 0.183 mol) slowly at 0 C, and the resulting mixture was stirred at room temperature for 2h. The reaction mixture was concentrated to half of the volume to afford the solid product which was filtered, washed with 10 ml of DCM and dried to provide 2-(2- chloro-acetylamino)-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid amide (35 g, 84%) as off-white solid. XH NMR (400 MHz, DMSO-d6): δ 1.65-1.75 (m, 4H), 2.58-2.72 (m, 4H), 4.49 (s, 2H), 7.29 (s, 1H), 7.42 (s, 1H), 12.23 (s, 1H); LC-MS: [M+H] = 272.8, 275.1, 257.9 & 255.9 m/z.
References:
WO2013/112651,2013,A2 Location in patent:Page/Page column 64

108-94-1
528 suppliers
$12.00/50g
![2-(2-CHLORO-ACETYLAMINO)-4,5,6,7-TETRAHYDRO-BENZO[B]THIOPHENE-3-CARBOXYLIC ACID AMIDE](/CAS/GIF/20886-87-7.gif)
20886-87-7
21 suppliers
$28.60/10MG
![ETHYL 2-AMINO-4,5,6,7-TETRAHYDROBENZO[B]THIOPHENE-3-CARBOXYLATE](/CAS/GIF/4506-71-2.gif)
4506-71-2
199 suppliers
$6.00/1g

79-04-9
352 suppliers
$12.00/5g
![2-(2-CHLORO-ACETYLAMINO)-4,5,6,7-TETRAHYDRO-BENZO[B]THIOPHENE-3-CARBOXYLIC ACID AMIDE](/CAS/GIF/20886-87-7.gif)
20886-87-7
21 suppliers
$28.60/10MG