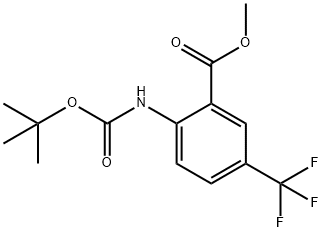
diMethylethoxy)carbonyl]aMino]-5-(trifluoroMethyl)-, Methyl ester synthesis
- Product Name:diMethylethoxy)carbonyl]aMino]-5-(trifluoroMethyl)-, Methyl ester
- CAS Number:209688-24-4
- Molecular formula:C14H16F3NO4
- Molecular Weight:319.28
117324-58-0
99 suppliers
$15.00/250mg

24424-99-5
825 suppliers
$13.50/25G
![diMethylethoxy)carbonyl]aMino]-5-(trifluoroMethyl)-, Methyl ester](/CAS/20180808/GIF/209688-24-4.gif)
209688-24-4
5 suppliers
inquiry
Yield:209688-24-4 49%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 72 h;
Steps:
139.2
Step 2. Preparation of Methyl-3-trifluoromethyl-2-t-butoxycarbonylaminobenzoate. Add di-t-butyl dicarbonate (2.0 g, 9.1 mmol) to a solution of methyl-3- trifluoromethyl-2-aminobenzoate (2.0 g, 9.1 mmol) in dichloromethane (20 mL). To this mixture, add triethylamine (9.1 mmol) and stir at room temperature under an atmosphere of nitrogen for 72 h. Dilute the reaction with dichloromethane (100 mL) and wash with water (100 mL x 2). Dry the separated organic phase over sodium sulfate, filter, and remove solvent under vacuum. Chromatograph the product on silica gel using hexane/ethyl acetate (gradient, 2-10 % ethyl acetate/hexane) to elute. This provides the title compound as a colorless solid (1.43 g, 49%) : H NMR (CDCl3, 400 MHz) 8 1.54 (s, 9H), 3.99 (s, 3H), 7.75 (dd, J = 2.0, 9.2 Hz, 1H), 8.30 (d, J = 1.6 Hz, 1H), 8.64 (d, J = 9.2 Hz), 10.48 (s, 1H).
References:
WO2005/37796,2005,A1 Location in patent:Page/Page column 138-139