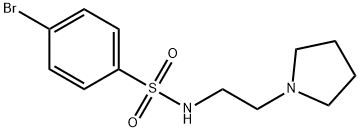
BenzenesulfonaMide, 4-broMo-N-[2-(1-pyrrolidinyl)ethyl]- synthesis
- Product Name:BenzenesulfonaMide, 4-broMo-N-[2-(1-pyrrolidinyl)ethyl]-
- CAS Number:209968-27-4
- Molecular formula:C12H17BrN2O2S
- Molecular Weight:333.24
Yield:209968-27-4 90%
Reaction Conditions:
with triethylamine in dichloromethane; for 3 h;
Steps:
9
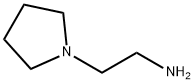
Example 9. 4-Broino-iV-(2-pvrrolidin-l-vl-ethvI)-benzenesulfonamide (6); [0139] 4-Bromo-benzenesulfonyl chloride (3.36 g, 13.1 mmol, 1 equiv) was dissolved in 50 niL DCM and treated with TEA (9.16 niL, 65.7 mmol, 5 equiv). To this, while stirring the solution, was added 2-pyrrolidm-l-yl-ethylamine (3 g, 26.3 mmol, 2 equiv). After 3 hours, reaction was poured onto DCM/water mixture and washed once. The aqueous phase was back extracted once with fresh DCM. Organic phases were combined, washed once with brine and dried over sodium sulfate. Filtration followed by rotary evaporation provided desired product. White needles (3.92 g, 90%). Rf = 0.35, 10% MeOH/ DCM.
References:
WO2006/101977,2006,A2 Location in patent:Page/Page column 41