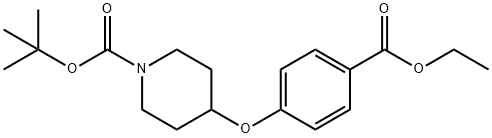
tert-Butyl 4-(4-(ethoxycarbonyl)phenoxy)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-(ethoxycarbonyl)phenoxy)piperidine-1-carboxylate
- CAS Number:210962-44-0
- Molecular formula:C19H27NO5
- Molecular Weight:349.42
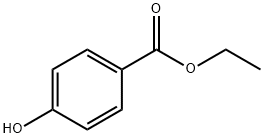
120-47-8
666 suppliers
$5.00/100mg

109384-19-2
469 suppliers
$5.00/1g

210962-44-0
25 suppliers
inquiry
Yield:210962-44-0 85.6%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in tetrahydrofuran;toluene at 0 - 20; for 24 h;
Steps:
1 Step 1
Step 1
To a solution of DEAD (12.3 mL, 27.08 mmol)(40% in toluene)was added to a mixture of ethyl 4-hydroxybenzoate (XIII)(3.0 g, 18.05 mmol), tert-butyl 4-hydroxypiperidine-1-carboxylate (XI)(4.72 g, 23.47 mmol)and triphenylphosphane (6.16 g, 23.47 mmol)in THF (40 mL)at 0° C.
The mixture was stirred from 0° C. to room temperature over 1 day before concentrating in vacuo.
The residue was diluted with EtOAc, washed with 1 N NaOH and brine, and then evaporated under vacuum.
The crude product was purified by chromatography (0→30% EtOAc/hexanes)to give tert-butyl 4-(4-ethoxycarbonylphenoxy)piperidine-1-carboxylate (XIV)(5.4 g, 15.45 mmol, 85.6% yield)as a colorless oil. ESIMS found for C19H27NO5 m/z 372.1 (M+Na).
References:
US2017/313682,2017,A1 Location in patent:Paragraph 0702; 0703

120-47-8
666 suppliers
$5.00/100mg

109384-19-2
469 suppliers
$5.00/1g

1972-28-7
381 suppliers
$15.00/5g

210962-44-0
25 suppliers
inquiry