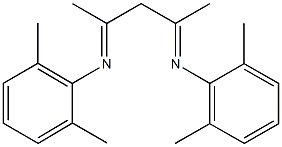
N-(2,6-dimethylphenyl)-N-{3-[(2,6-dimethylphenyl)imino]-1-methylbutylidene}amine synthesis
- Product Name:N-(2,6-dimethylphenyl)-N-{3-[(2,6-dimethylphenyl)imino]-1-methylbutylidene}amine
- CAS Number:213130-55-3
- Molecular formula:C21H26N2
- Molecular Weight:306.4445
Yield:213130-55-3 83%
Reaction Conditions:
with toluene-4-sulfonic acid in benzene; for 24 h;Schlenk technique;Inert atmosphere;Reflux;
Steps:
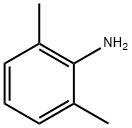
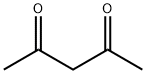
General procedure: For ligand B, 4.4 mL (42.8 mmol) of acetylacetone,11.1 mL of 2,4-dimethylaniline (89.9 mmol) and 42.8 mmol ofp-toluenesulfonic acid in 100 mL of benzene were added to aSchlenk tube. For ligand C, 5.7 mL (55.5 mmol) of acetylacetone,16.4 mL (116.6 mmol) of 2,4,6-trimethylaniline and 55.5 mmolof p-toluenesulfonic acid in 100 mL of benzene were added toa Schlenk tube. In both cases, the suspension was refluxed for24 h, and the water formed was removed using the Dean-Starkapparatus. The solvent was removed under reduced pressure.Then, 60 mL of CH2Cl2 and 80 mL of a saturated solution of sodiumcarbonate were added. The aqueous phase was extracted withCH2Cl2. After the solvent was evaporated under reduced pressure,the solids were recrystallized with methanol to give 35.5 mmol of B and 39.5 mmol of C, which corresponded to 83% and 92% yields,respectively
References:
Rossetto, Enéderson;Caovilla, Marcela;Thiele, Daniel;De Souza, Roberto F.;Bernardo-Gusm?o, Katia [Applied Catalysis A: General,2013,vol. 454,p. 152 - 159]