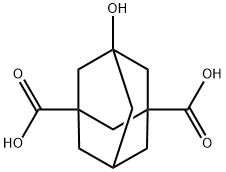
Tricyclo[3.3.1.13,7]decane-1,3-dicarboxylic acid, 5-hydroxy- synthesis
- Product Name:Tricyclo[3.3.1.13,7]decane-1,3-dicarboxylic acid, 5-hydroxy-
- CAS Number:213274-55-6
- Molecular formula:C12H16O5
- Molecular Weight:240.25

39269-10-8
188 suppliers
$9.00/1g
![Tricyclo[3.3.1.13,7]decane-1,3-dicarboxylic acid, 5-hydroxy-](/CAS/20210111/GIF/213274-55-6.gif)
213274-55-6
0 suppliers
inquiry
Yield:213274-55-6 75.5%
Reaction Conditions:
with sulfuric acid;nitric acid at 35 - 50; for 5.5 h;Temperature;
Steps:
1
As a reaction apparatus, a stirrer,thermometer,A dropping funnel,Into a glass flask equipped with a Dimroth condenser,100.0 g of 1,3-adamantanedicarboxylic acid (purity of 99% referred to as ADCA)900 g of 96 mass% concentrated sulfuric acid (9 mass times with respect to the raw material) was added.After stirring at 35 ° C. to confirm dissolution of the raw material,Cool the Lasco and keep the liquid temperature at 35 to 50 ° C120 g of 70% by mass nitric acid was added dropwise over 30 minutes so as to stay within the range.After completion of the dropwise addition,The reaction was carried out at a reaction temperature of 50 ° C. for 5 hours.When the progress of the reaction was confirmed with a gas chromatogram (GC)The conversion of 1,3-adamantane dicarboxylic acid was 100%With a GC peak area ratio of 98.0%5-hydroxyadamantane-1,3-dicarboxylic acid was formed. A stirrer,To a reaction stopping apparatus equipped with a glass flask equipped with a thermometer and an alkali trap,900 g of ion exchange water (pure water) was added.next,While cooling the flask,In order to keep the liquid temperature within the range of 10 to 40 ° C.,The reaction solution of 5-hydroxyadamantane-1,3-dicarboxylic acid was added dropwise to terminate the reaction to precipitate crystals of the objective substance.The total amount of the reaction stopped solution after completion of dropping of the reaction solution was 2020 g,It was 20 times the charged mass of 1,3-adamantane dicarboxylic acid.Incidentally,The pH of the reaction stopped solution was <0.The precipitated crystals were separated by filtration,It was washed with water.The obtained crystals were dried under reduced pressure at 40 ° C. for 8 hours,80.7 g (yield 75.5%) of white crystal of 5-hydroxyadamantane-1,3-dicarboxylic acid was obtained.
References:
JP6024410,2016,B2 Location in patent:Paragraph 0032; 0033
5001-18-3
328 suppliers
$6.00/1g
![Tricyclo[3.3.1.13,7]decane-1,3-dicarboxylic acid, 5-hydroxy-](/CAS/20210111/GIF/213274-55-6.gif)
213274-55-6
0 suppliers
inquiry