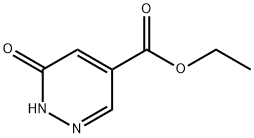
6-oxo-1,6-dihydropyridazine-4-carboxylic acid ethyl ester synthesis
- Product Name:6-oxo-1,6-dihydropyridazine-4-carboxylic acid ethyl ester
- CAS Number:21427-85-0
- Molecular formula:C7H8N2O3
- Molecular Weight:168.15
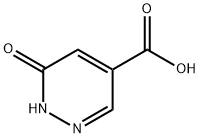
867130-58-3
96 suppliers
$37.00/250mg

64-17-5
704 suppliers
$10.00/50g

21427-85-0
18 suppliers
inquiry
Yield:21427-85-0 83%
Reaction Conditions:
Stage #1: 6-oxo-1,6-dihydro-pyridazine-4-carboxylic acid;ethanolwith sulfuric acid for 5 h;Heating / reflux;
Stage #2: with water;sodium carbonate
Steps:
14.C
Step 14C: Ethyl 6-oxo-1H-pyridazine-4-carboxylate; The compound of Step 14B was dissolved in EtOH (10 mL) and concentrated H2SO4 (4.2 mL) was added and then heated at reflux for 5 h. The reaction mixture was cooled, concentrated in vacuo and basified with saturated Na2CO2. After filtration, the aqueous phase was extracted with EtOAc, dried over anhydrous Na2SO4, filtered and concentrated to give the subtitle product (83%).1H NMR (400 MHz, CD3OD): δ 8.27 (d, 1H), 7.42 (d, 1H), 4.40 (q, 2H), 1.39 (t, 3H).
References:
US2009/111820,2009,A1 Location in patent:Page/Page column 22
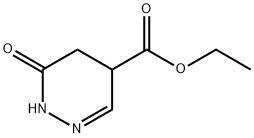
412301-44-1
0 suppliers
inquiry

21427-85-0
18 suppliers
inquiry
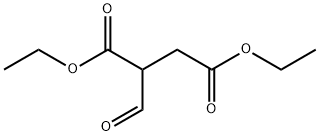
5472-38-8
33 suppliers
$65.00/100mg

21427-85-0
18 suppliers
inquiry