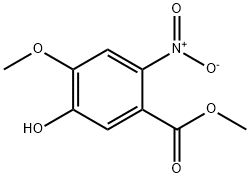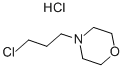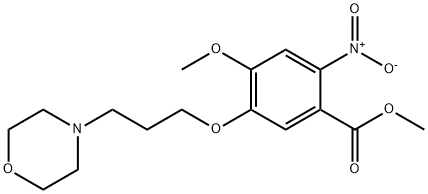
Methyl 4-Methoxy-5-(3-Morpholinopropoxy)-2-nitrobenzoate synthesis
- Product Name:Methyl 4-Methoxy-5-(3-Morpholinopropoxy)-2-nitrobenzoate
- CAS Number:214472-37-4
- Molecular formula:C16H22N2O7
- Molecular Weight:354.36
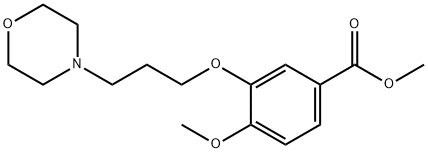
214472-17-0
1 suppliers
inquiry

214472-37-4
18 suppliers
inquiry
Yield:214472-37-4 92.3%
Reaction Conditions:
with sulfuric acid;nitric acid;sodium hydroxide in water;acetic acid at 5 - 20;
Steps:
II.1
(1) Preparation of methyl 2-nitro-4-methoxy-5-(3-morpholinopropoxy)benzoate 4 Methyl 4-methoxy-3-(3-morpholinopropoxy)benzoate 3 (43.5 g, 0.1408 mol) is dissolved in acetic acid (117 ml) at normal temperature, after moving to an environment with 5° C. for ten minutes, HNO3 (21.75 ml, 45.5%) is added to react for 30 minutes, H2SO4 (44 ml, 70%) is added, and after cooled to room temperature, reaction is carried out for 2 hours. After reaction is completed, ice water (300 ml) is added at 5° C., water (170 ml) is used to wash a bottle with a rounded bottom, alkalinity is adjusted by adding NaOH (280 ml, 50%), stirring is carried out for 1 hours at a temperature less than 5° C., and extraction is performed by using ethyl acetate (770 ml) for twice. Extracts of ethyl acetate layer are combined, to be dried by MgSO4, filtered, concentrated and dried for 16 hours to obtain daffadilly solid compound 4 (46 g, 92.3%).
References:
US2010/267949,2010,A1 Location in patent:Page/Page column 6
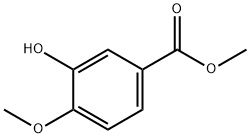
6702-50-7
193 suppliers
$11.00/1g

214472-37-4
18 suppliers
inquiry
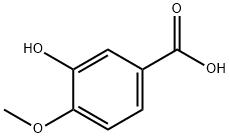
645-08-9
329 suppliers
$10.00/1g

214472-37-4
18 suppliers
inquiry