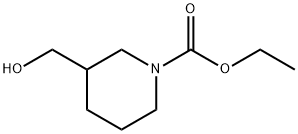
1-Piperidinecarboxylic acid, 3-(hydroxymethyl)-, ethyl ester synthesis
- Product Name:1-Piperidinecarboxylic acid, 3-(hydroxymethyl)-, ethyl ester
- CAS Number:214548-40-0
- Molecular formula:C9H17NO3
- Molecular Weight:187.24
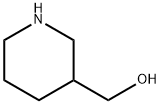
4606-65-9
253 suppliers
$9.00/1g
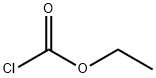
541-41-3
6 suppliers
$23.00/5g
497-19-8
1384 suppliers
$14.00/1KG

214548-40-0
15 suppliers
$237.00/500mg
Yield:214548-40-0 110 g (98%)
Reaction Conditions:
in chloroform;water;
Steps:
A.6.a EXAMPLE A6
a) A mixture of 3-hydroxymethyl-piperidine (0.6 mol) and Na2 CO3 (130 g) in CHCl3 (600 ml) and water (600 ml) was stirred at 10° C. Ethyl chloroformate (115 g) was added dropwise (temperature was kept at 10° C.). The mixture was stirred until the temperature reached room temperature and the reaction mixture was stirred overnight. Water (500 ml) was added. The organic layer was separated, washed with water, dried, filtered and the solvent was evaporated, yielding 110 g (98%) of (+-)-ethyl 3-(hydroxymethyl)-1-piperidinecarboxylate (interm. 17).
References:
US6156757,2000,A

4606-65-9
253 suppliers
$9.00/1g
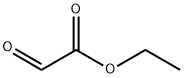
924-44-7
264 suppliers
$12.00/5g

214548-40-0
15 suppliers
$237.00/500mg

4606-65-9
253 suppliers
$9.00/1g

541-41-3
6 suppliers
$23.00/5g

214548-40-0
15 suppliers
$237.00/500mg