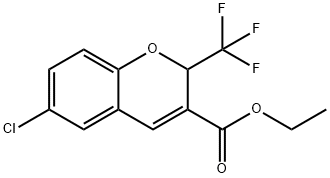
Ethyl 6-chloro-2-(trifluoromethyl)-2H-chromene-3-carboxylate synthesis
- Product Name:Ethyl 6-chloro-2-(trifluoromethyl)-2H-chromene-3-carboxylate
- CAS Number:215123-85-6
- Molecular formula:C13H10ClF3O3
- Molecular Weight:306.66

25597-16-4
226 suppliers
$7.00/1g

635-93-8
347 suppliers
$6.00/5g

215123-85-6
7 suppliers
$181.00/500mg
Yield:215123-85-6 62%
Reaction Conditions:
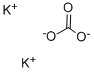
with potassium carbonate in DMF (N,N-dimethyl-formamide) at 60; for 20 h;
Steps:
44.1 Example 44; 6-CHLORO-8- (METHYLTHIO)-2- (TRIFLUOROMETHYL)-2H-CHROMENE-3-CARBOXYLIC acid; Step 1. Preparation of ethyl 6-chloro-2-(trifluoromethyl)-2H-chromene-3-carboxylate.
[0511] 5-Chlorosalicylaldehyde (20.02 g, 0.128 mole) and ethyl 4,4, 4-trifluorocrotonate (23.68 g, 0.14 mole) were dissolved in anhydrous DMF, warmed to 60 °C and treated with anhydrous K2CO3 (17.75 g, 0. 128 mole). The solution was maintained at 60 °C for 20 hours, cooled to room temperature, and diluted with water. The solution was extracted with ethyl acetate. The combined extracts were washed with brine, dried over anhydrous MGS04, filtered and concentrated in vacuo to afford 54.32 g of an oil. The oil was dissolved in 250 mL of methanol and 100 mL of water, whereupon a white solid formed that was isolated by filtration. The resulting solid was washed with water and dried in vacuo, to afford the ester as a yellow solid (24.31 g, 62%): mp 62-64 °C. 1H NMR (CDC13/90 MHz) 7.64 (s, 1H), 7.30-7. 21 (m, 2H), 6.96 (d, 1H, J= Hz), 5.70 (q, 1H, J= Hz), 4.30 (q, 2H, J= 7.2 Hz), 1.35 (t, 3H, J=7.2 Hz).
References:
WO2004/87686,2004,A2 Location in patent:Page 244-245 WO2004/87687,2004,A1 Location in patent:Page 244-245

25597-16-4
226 suppliers
$7.00/1g
584-08-7
1182 suppliers
$5.00/100g

635-93-8
347 suppliers
$6.00/5g

215123-85-6
7 suppliers
$181.00/500mg