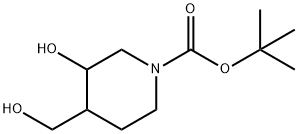
tert-butyl 3-hydroxy-4-(hydroxyMethyl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 3-hydroxy-4-(hydroxyMethyl)piperidine-1-carboxylate
- CAS Number:220218-58-6
- Molecular formula:C11H21NO4
- Molecular Weight:231.29
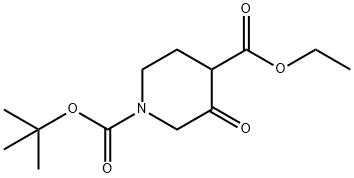
71233-25-5
150 suppliers
$6.00/1g

220218-58-6
19 suppliers
$350.00/100mg
Yield:220218-58-6 86%
Reaction Conditions:
with sodium borohydrid in methanol;water;
Steps:
33.B tert-butyl 3-hydroxy-4-(hydroxymethyl)-1-piperidinecarboxylate
EXAMPLE 33B tert-butyl 3-hydroxy-4-(hydroxymethyl)-1-piperidinecarboxylate The product from Example 33A (10.84 g, 40 mmol) in methanol (200 mL) was treated with sodium borohydride (9.12 g, 240 mmol) slowly over 20 minutes at 0-10° C. The mixture was allowed to warm to ambient temperature and stirred for 20 hours. The mixture was concentrated under reduced pressure, treated with water (50 mL), and extracted with chloroform (2*10 mL). The organic phase was concentrated under reduced pressure to provide the title compound (8.0 g, 86% yield). 1H NMR (CD3OD, 300 MHz) δ 1.48 (s, 9H), 1.70-1.50 (m, 3H), 2.90-2.70 (m, 2H), 3.72-3.40 (m, 2H), 4.18-3.90 (m, 4H); MS (DCI/NH3) m/z 232 (M+H)+.
References:
US2002/19388,2002,A1
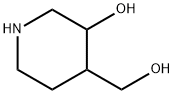
220218-57-5
12 suppliers
inquiry

24424-99-5
821 suppliers
$13.50/25G

220218-58-6
19 suppliers
$350.00/100mg
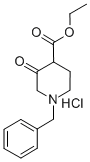
52763-21-0
241 suppliers
$6.00/1g

220218-58-6
19 suppliers
$350.00/100mg
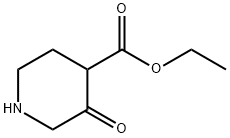
70637-75-1
38 suppliers
$90.00/50mg

220218-58-6
19 suppliers
$350.00/100mg