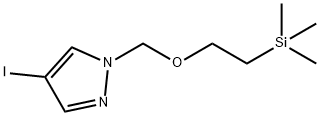
1H-Pyrazole, 4-iodo-1-[[2-(triMethylsilyl)ethoxy]Methyl]- synthesis
- Product Name:1H-Pyrazole, 4-iodo-1-[[2-(triMethylsilyl)ethoxy]Methyl]-
- CAS Number:220299-49-0
- Molecular formula:C9H17IN2OSi
- Molecular Weight:324.23
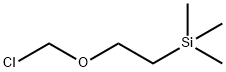
76513-69-4
351 suppliers
$6.00/1g
![1H-Pyrazole, 4-iodo-1-[[2-(triMethylsilyl)ethoxy]Methyl]-](/CAS/GIF/220299-49-0.gif)
220299-49-0
10 suppliers
inquiry
Yield:220299-49-0 100%
Reaction Conditions:
Stage #1: 4-iodopyrazolewith sodium hydride in tetrahydrofuran at 20; for 0.666667 h;
Stage #2: (2-trimethylethylsilylethoxy)methyl chloride in tetrahydrofuran at 20; for 4.53333 h;
Steps:
42.a
EXAMPLE 42; Preparation of 2-Amino-5-(1-ethyl-1H-pyrazol-4-yl)-3-methyl-5-(3-pyrimidin-5-yl)-3,5-dihydro-4H-imidazol-4-one; Step a); Preparation of Compound 10; Sodium hydride (0.11 g of a 60% dispersion in oil, 2.84 mmol) was washed with hexanes, diluted with THF (2 mL) and treated with a solution of 9 (0.50 g, 2.58 mmol) in THF (1 mL) over a period of 5 min. After stirring for 35 min at room temperature, a solution of 2-(trimethylsilyl)ethoxymethyl chloride (0.52 g, 3.10 mmol) in THF (1 mL) was added dropwise over a period of 2 min and the mixture stirred at room temperature for 4.5 h. The mixture was diluted with ether (20 mL) then washed with water (10 mL), brine 10 mL), dried over magnesium sulfate, filtered and concentrated to afford 10 (0.85 g, 100%) as an amber oil: 1H NMR (500 MHz, CDCl3) δ 7.65 (s, 1H), 7.55 (s, 1H), 5.41 (s, 2H), 3.58 (m, 2H), 0.91 (m, 2H), 0.50 (s, 9H); ESI MS m/z 325 [C9H17IN2 OSi+H]+.
References:
US2007/4786,2007,A1 Location in patent:Page/Page column 22

76513-69-4
351 suppliers
$6.00/1g
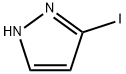
4522-35-4
123 suppliers
$9.00/250mg
![1H-Pyrazole, 4-iodo-1-[[2-(triMethylsilyl)ethoxy]Methyl]-](/CAS/GIF/220299-49-0.gif)
220299-49-0
10 suppliers
inquiry
