Tetrabutyl phosphonium salt with 1,1,2,2,3,3,4,4,4-nonafluoro-1-butanesulfonic acid(1:1) synthesis
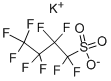
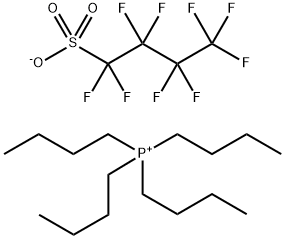
- Product Name:Tetrabutyl phosphonium salt with 1,1,2,2,3,3,4,4,4-nonafluoro-1-butanesulfonic acid(1:1)
- CAS Number:220689-12-3
- Molecular formula:C20H36F9O3PS
- Molecular Weight:558.52
Yield:220689-12-3 89.1%
Reaction Conditions:
in ethanol;water at 20; for 0.25 h;Product distribution / selectivity;
Steps:
3
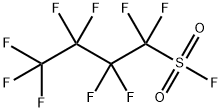
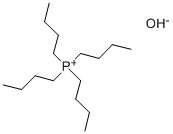
Comparative Example 3; Preparation of TBPPFS using perfluorobutane sulfonyl fluoride and tetrabutylphosphoniumbromide in ETOH/H2O at RT (20° C.). A portion of K Rimar (6.06 gram, 17.9 mmol) was dissolved at room temperature (20° C.) in 75 ml of an EtOH/MQ water mixture (volume ratio EtOH:MQ water=3:4). Separately, TBPBr (6.01 g, 17.7 mmol) was dissolved in 25 ml of MQ water, and was subsequently poured gradually into the solution of K Rimar, with stirring. After addition, the reaction mixture was stirred for an additional 15 minutes. The target product was extracted with 75 ml of dichloromethane, which was in turn washed three times with 50 ml of MQ water. The organic layer solvent was removed by rotary evaporation (50° C. 125 mbar), and the resulting white solid was dried overnight at 50° C. under reduced pressure. Further purification was done by dispersing the isolated white powder in 100 ml MQ water and heat the dispersion up to 80° C. with stirring. Stirring was continued for 5 minutes and a hazy solution was observed. The dispersion was then cooled to room temperature (20° C.) and a solid white material crystallized. This white material was isolated and dried overnight at 50° C. under reduced pressure. Yield: 89.1%; Mp: 75.6° C.
References:
US2008/15377,2008,A1 Location in patent:Page/Page column 6
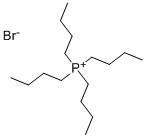
3115-68-2
300 suppliers
$10.00/5g
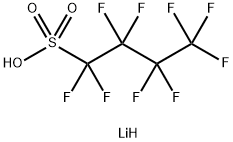
131651-65-5
124 suppliers
$17.00/5g

220689-12-3
3 suppliers
inquiry