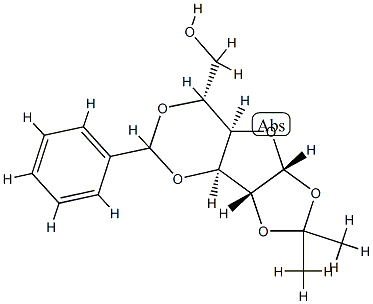
3-O,5-O-Benzylidene-1-O,2-O-isopropylidene-α-D-glucofuranose synthesis
- Product Name:3-O,5-O-Benzylidene-1-O,2-O-isopropylidene-α-D-glucofuranose
- CAS Number:22164-09-6
- Molecular formula:C16H20O6
- Molecular Weight:308.3264
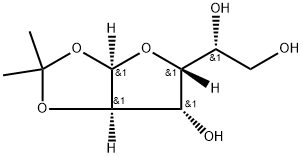
18549-40-1
357 suppliers
$10.00/5g

22164-09-6
6 suppliers
inquiry
Yield:22164-09-6 56%
Reaction Conditions:
with benzaldehyde;Zinc chloride
Steps:
1 Synthesis of 3,5-O-benzylidene-1,2-O-isopropylidene-α-D-glucofuranoside
EXAMPLE 1 Synthesis of 3,5-O-benzylidene-1,2-O-isopropylidene-α-D-glucofuranoside To a mixture of 1,2-O-isopropylidene-α-D-glucofuranoside (3.0 g, 13.6 mmol) and anhydrous zinc chloride (4.0 g, 29.4 mmol) was added benzaldehyde (12.0 g, 114 mmol) at room temperature under nitrogen. The mixture was stirred for 4 hours, then diluted with ethyl acetate (EtOAc, 20 mL). The resulting solution was washed with water (2*40 mL), evaporated, and recrystallized from hexane at 0° C. to afford 3,5-O-benzylidene-1,2-O-isopropylidene-α-D-glucofuranoside (2.48 g, 56%) as a white solid. 1H NMR (300 MHz, CDCl3) δ7.47 (m, 2H), 7.36 (m, 3H), 6.03 (d, 1H, J=3.6 Hz), 5.84 (s, 1H), 4.65 (d, 1H, J=3.6 Hz), 4.46 (d, 1H, J=2.2 Hz), 4.36 (dd, 1H, J=7.1, 4.5 Hz), 4.11 (d, 1H, J=2.2 Hz), 4.06 (ddd, 1H, J=11.6, 7.1, 4.5 Hz), 3.91 (ddd, 1H, J=11.6, 7.1, 4.5 Hz), 1.86 (dd, 1H, J=7.1, 3.6 Hz), 1.51 (s, 3H), 1.32 (s, 3H); 13C NMR (75 MHz, CDCl3) δ137.5 (C), 129.3 (CH), 128.3 (CH), 126.2 (CH), 111.9 (C), 104.9 (CH), 94.4 (CH), 83.8 (CH), 78.0 (CH), 74.0 (CH), 72.9 (CH), 61.9 (CH2), 26.6 (CH3), 26.0 (CH3).
References:
US6294666,2001,B1

32754-29-3
2 suppliers
inquiry

22164-09-6
6 suppliers
inquiry

100-52-7
943 suppliers
$20.37/250g

18549-40-1
357 suppliers
$10.00/5g
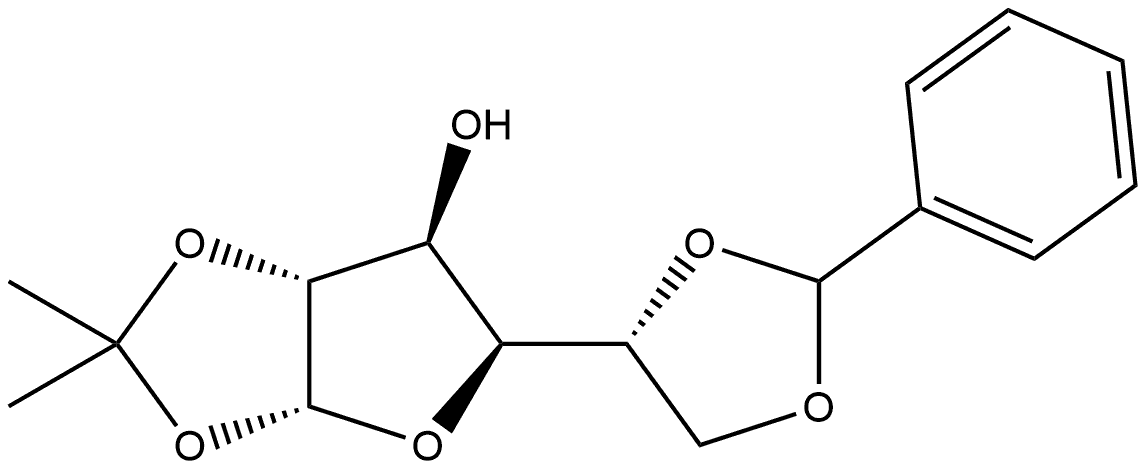
23397-77-5
0 suppliers
inquiry

22164-09-6
6 suppliers
inquiry

100-52-7
943 suppliers
$20.37/250g

18549-40-1
357 suppliers
$10.00/5g

22164-09-6
6 suppliers
inquiry