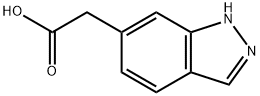
2-(1H-Indazol-6-yl)ethanoic acid, 6-(Carboxymethyl)-1H-indazole synthesis
- Product Name:2-(1H-Indazol-6-yl)ethanoic acid, 6-(Carboxymethyl)-1H-indazole
- CAS Number:221681-76-1
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
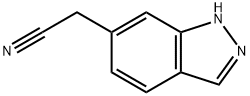
1146323-46-7
6 suppliers
inquiry

221681-76-1
11 suppliers
inquiry
Yield:221681-76-1 96%
Reaction Conditions:
Stage #1: (1H-indazole-6-yl)-acetonitrilewith sodium hydroxide;water for 2 h;Heating / reflux;
Stage #2: with hydrogenchloride in water;
Steps:
4
A solution of the starting (lH-indazole-6-yl)acetonitrile (27.0 mg, 0.172 mmol) in 0.1 N NaOH (859.0 μl, 0.859 mmol) was refluxed under nitrogen for 2.0 h. The mixture was cooled and titrated with 10% hydrochloric acid. A white solid precipitated. It was filtered, washed with water. The solid was taken with 10.0 ml of ethyl acetate, dried over MgSO4, filtered and concentrated to give the crude title compound as a white solid (0.029 g, 96%). 1H NMR (CD3OD, 400 MHz) δ 8.006 (s, IH); 7.709 (d, J= 8.33 Hz, IH); 7.460 (s, IH); 7.094 (dd, J= 8.53 Hz, 1.19 Hz, IH); 3.740 (s, 2H).
References:
WO2009/54983,2009,A1 Location in patent:Page/Page column 38-39