
methyl trans-4-({[(tert-butoxy)carbonyl]amino}methyl)cyclohexane-1-carboxylate synthesis
- Product Name:methyl trans-4-({[(tert-butoxy)carbonyl]amino}methyl)cyclohexane-1-carboxylate
- CAS Number:222986-86-9
- Molecular formula:C14H25NO4
- Molecular Weight:271.35
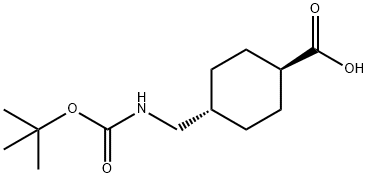
27687-14-5
108 suppliers
$10.00/1g

74-88-4
344 suppliers
$15.00/10g
![methyl trans-4-({[(tert-butoxy)carbonyl]amino}methyl)cyclohexane-1-carboxylate](/CAS/20180703/GIF/222986-86-9.gif)
222986-86-9
21 suppliers
$293.00/25g
Yield:222986-86-9 94.89 %
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20;Inert atmosphere;
Steps:
1.2 Step 2: Synthesis of trans-methyl 4-(((tert-butoxycarbonyl)amino)methyl)cyclohexane-1-carboxylate
[0336] To a stirred solution of trans-methyl 4-(((tert-butoxycarbonyl)amino)methyl)cyclohexane-1-carboxylate (800 mg, 3.11 mmol, 1 eq.) in DMF (5 mL) was added K2CO3 (1288 mg, 9.336 mmol, 2 eq.) and methyl iodide (0.6 mL, 9.336 mmol, 1 eq.) The resulting reaction mixture was stirred at RT for 3 hours under nitrogen atmosphere. Product formation was confirmed by TLC and LCMS. Upon completion, the reaction mixture was poured into ice cold water (10 ml). The resulting solid was filtered off and dried under vacuum to obtain trans-methyl 4-(((tert-butoxycarbonyl)amino)methyl)cyclohexane-1-carboxylate (800 mg, 94.89 % yield) as a brown solid.1H NMR (400 MHz, DMSO-d6) G 6.80 (t, J = 5.7 Hz, 1H), 3.57 (s, 3 H), 2.81 - 2.68 (m, 2 H), 2.28 - 2.12 (m, 1H), 2.01 - 1.77 (m, 2 H), 1.69 (d, J = 10.5 Hz, 3 H), 1.42 - 1.32 (m, 9 H), 1.32 - 1.10 (m, 2 H), 1.00 - 0.81 (m, 2 H).
References:
WO2022/212902,2022,A1 Location in patent:Paragraph 0335-0336

24424-99-5
823 suppliers
$13.50/25G
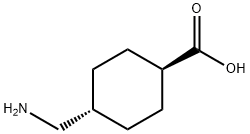
1197-18-8
822 suppliers
$10.00/5g
![methyl trans-4-({[(tert-butoxy)carbonyl]amino}methyl)cyclohexane-1-carboxylate](/CAS/20180703/GIF/222986-86-9.gif)
222986-86-9
21 suppliers
$293.00/25g

24424-99-5
823 suppliers
$13.50/25G
![methyl trans-4-({[(tert-butoxy)carbonyl]amino}methyl)cyclohexane-1-carboxylate](/CAS/20180703/GIF/222986-86-9.gif)
222986-86-9
21 suppliers
$293.00/25g

1197-18-8
822 suppliers
$10.00/5g
![methyl trans-4-({[(tert-butoxy)carbonyl]amino}methyl)cyclohexane-1-carboxylate](/CAS/20180703/GIF/222986-86-9.gif)
222986-86-9
21 suppliers
$293.00/25g