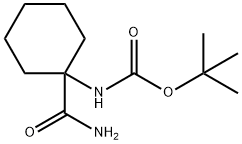
(1-Carbamoyl-cyclohexyl)-carbamic acid tert-butyl ester synthesis
- Product Name:(1-Carbamoyl-cyclohexyl)-carbamic acid tert-butyl ester
- CAS Number:223648-39-3
- Molecular formula:C12H22N2O3
- Molecular Weight:242.31
115951-16-1
140 suppliers
$11.19/1G

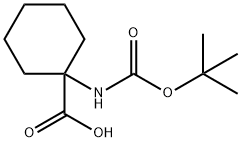
223648-39-3
8 suppliers
$424.00/250mg
Yield:223648-39-3 84%
Reaction Conditions:
with 4-methyl-morpholine;ammonium chloride;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;3-hydroxy-3,4-dihydrobenzotriazine-4-one in DMF (N,N-dimethyl-formamide) at 20; for 70 h;
Steps:
1
Step 2 Compound 5013 was prepared from 5013a according to the procedures described for the preparation of compound 5000. Preparation of compound of formula 5014 Step 1 To the suspension of 5014a (10.0 g, 41.1 mmol), HOOBt (8.7 g, 53.3 mmol), EDCI (10.0 g, 52.2 mmol) and ammonium chloride (8.90 g, 166 mmol) in anhydrous DMF (400 mL) at rt was added 4-methylmorpholine (22.5 mL, 204.5 mmol). The mixture was stirred at rt for 70 h. Brine (150 mL) and 5% aqueous phosphoric acid solution (150 mL) were added followed by EtOAc (800 mL). The layers were separated and the organic solution was washed with 5% aqueous phosphoric acid solution (400 mL) and saturated sodium bicarbonate solution (2 x 400 mL) before it was dried, filtered and concentrated to give 8.35 g product 5014b (84%).
References:
WO2005/85242,2005,A1 Location in patent:Page/Page column 91