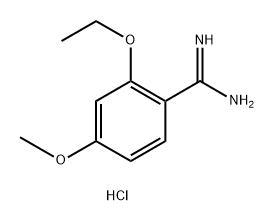
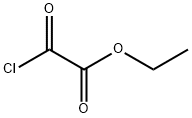
Imidazo[5,1-f][1,2,4]triazin-4(1H)-one, 2-(2-ethoxyphenyl)-5,7-dimethyl- synthesis
- Product Name:Imidazo[5,1-f][1,2,4]triazin-4(1H)-one, 2-(2-ethoxyphenyl)-5,7-dimethyl-
- CAS Number:224789-20-2
- Molecular formula:C15H16N4O2
- Molecular Weight:284.31
![N-[1-[3-(2-Ethoxyphenyl)-2,5-dihydro-5-oxo-1,2,4-triazin-6-yl]ethyl]acetamide](/CAS/20200401/GIF/CB75161381.gif)
1417529-59-9
13 suppliers
inquiry
![Imidazo[5,1-f][1,2,4]triazin-4(1H)-one, 2-(2-ethoxyphenyl)-5,7-dimethyl-](/CAS/20200611/GIF/224789-20-2.gif)
224789-20-2
16 suppliers
inquiry
Yield:224789-20-2 41%
Reaction Conditions:
with trichlorophosphate in toluene; for 2 h;Reflux;
Steps:
1 General procedure for the synthesis of the 2(2-ethoxy-phenyl)-7-alkyl-5-methyl-imidazo[5,1-f][1,2,4]triazin-4(3H)-one 14
General procedure: To a magnetically stirred solution of 13 (0.0010 mol) in toluene (35 mL), phosphorus oxychloride (0.17 mL, 0.0019 mol) was added at room temperature. The resulting mixture was heated under reflux for 2 h and then cooled to room temperature. The solvent and an excess of phosphorus oxychloride were evaporated in vacuo and the residue was treated with saturated aqueous sodium bicarbonate solution (15 mL) and chloroform (15 mL). The mixture was shaken vigorously until all solid had dissolved. The chloroform layer was separated and the aqueous phase was extracted with another portion of chloroform (2 15 mL). The chloroform extracts were combined, dried (MgSO4), filtered, and the solvent was evaporated. The residue was purified by column chromatography on silica gel (chloroform) afforded a product, which was then crystallized from an appropriate solvent.
References:
Olszewska, Teresa;Gajewska, Ewa P.;Milewska, Maria J. [Tetrahedron,2013,vol. 69,# 2,p. 474 - 480]
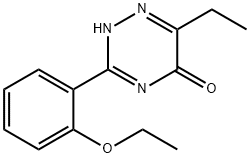
1417529-52-2
0 suppliers
inquiry
![Imidazo[5,1-f][1,2,4]triazin-4(1H)-one, 2-(2-ethoxyphenyl)-5,7-dimethyl-](/CAS/20200611/GIF/224789-20-2.gif)
224789-20-2
16 suppliers
inquiry
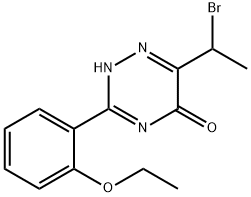
1417529-54-4
5 suppliers
inquiry
![Imidazo[5,1-f][1,2,4]triazin-4(1H)-one, 2-(2-ethoxyphenyl)-5,7-dimethyl-](/CAS/20200611/GIF/224789-20-2.gif)
224789-20-2
16 suppliers
inquiry
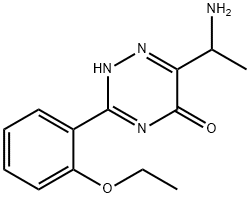
1417529-56-6
3 suppliers
inquiry
![Imidazo[5,1-f][1,2,4]triazin-4(1H)-one, 2-(2-ethoxyphenyl)-5,7-dimethyl-](/CAS/20200611/GIF/224789-20-2.gif)
224789-20-2
16 suppliers
inquiry