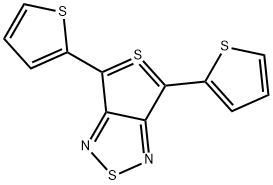
Thieno[3,4-c][1,2,5]thiadiazole-5-SIV, 4,6-di-2-thienyl- (9CI) synthesis
- Product Name:Thieno[3,4-c][1,2,5]thiadiazole-5-SIV, 4,6-di-2-thienyl- (9CI)
- CAS Number:225114-99-8
- Molecular formula:C12H6N2S4
- Molecular Weight:306.43
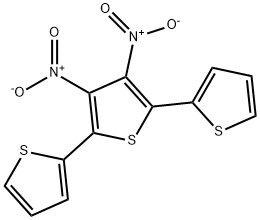
205170-72-5
14 suppliers
inquiry
![Thieno[3,4-c][1,2,5]thiadiazole-5-SIV, 4,6-di-2-thienyl- (9CI)](/CAS/20211123/GIF/225114-99-8.gif)
225114-99-8
1 suppliers
inquiry
Yield:225114-99-8 90%
Reaction Conditions:
Stage #1: 3',4'-dinitro-2,2':5',2''-terthiophenewith ammonia hydrochloride;zinc in methanol;dichloromethane;lithium hydroxide monohydrate at 20; for 2 h;Inert atmosphere;
Stage #2: with pyridine;chloro-trimethyl-silane at 20; for 2 h;Inert atmosphere;
Steps:
1.1 Step 1: Preparation of compound 3a
Compound 2a (2g, 5.9mmol), zinc powder (13.8g, 212.4mmol) and ammonium chloride (18.8g, 354mmol) were added to a 500mL round-bottom flask, methanol - water (v/v, 9:1) 100mL and dichloromethane 100mL were added under argon protection, and argon bubbling into the reaction solution for 5min to exclude oxygen in the system, argon protection was reacted at room temperature for 2 hours. After the end of the reaction, it was cooled to room temperature, methanol was removed by rotary steaming, the residue was re-dissolved in 150mL dichloromethane, water (30mL×3) washed three times, saturated table salt water (30mL×3) washed three times. The organic phase was dried with anhydrous magnesium sulfate for 3 hours, filtered, and the filtrate was spun dry to obtain intermediates. Take the above intermediates, N-sulfonylaniline (2.47g, 17.8mmol) and trimethylchlorosilane (2.57g, 23.7mmol) added to the 50mL round bottom flask, 20mL of pyridine was added under the protection of argon, and the argon bubble was injected into the reaction solution for 5min to eliminate oxygen in the system, and the reaction was reacted at room temperature for 2 hours under the protection of argon. After the reaction, it was cooled to room temperature, pyridine was removed by rotary steam, the residue was re-dissolved in 150mL dichloromethane, washed three times with water (30mL×3) and washed three times with saturated saline (30mL×3). The organic phase was dried for 3 hours with anhydrous magnesium sulfate, filtered, and the filtrate was spun dry to give 1.62g of compound 3a, yield: 90%
References:
CN114805396,2022,A Location in patent:Paragraph 0065-0066; 0070-0076
![[2,2':5',2''-Terthiophene]-3',4'-diaMine](/CAS/20150408/GIF/185691-91-2.gif)
185691-91-2
35 suppliers
inquiry
![Thieno[3,4-c][1,2,5]thiadiazole-5-SIV, 4,6-di-2-thienyl- (9CI)](/CAS/20211123/GIF/225114-99-8.gif)
225114-99-8
1 suppliers
inquiry