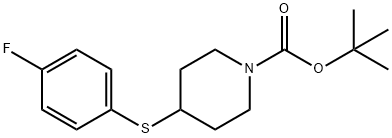
4-(4-Fluoro-phenylsulfanyl)-piperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-(4-Fluoro-phenylsulfanyl)-piperidine-1-carboxylic acid tert-butyl ester
- CAS Number:226398-48-7
- Molecular formula:C16H22FNO2S
- Molecular Weight:311.41
Yield:226398-48-7 100%
Reaction Conditions:
with potassium carbonate in acetonitrile; for 18 h;Heating / reflux;
Steps:
18.B
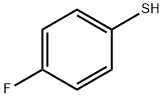
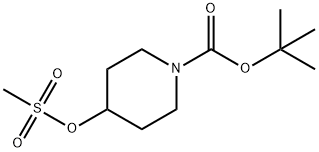
Step B: tert-Butyl 4-(4-fluoro-phenylsulfanyl)-piperidine- 1 -carboxylateTo a solution of the product from Step A (5.30 g, 18.99 mmol) in acetonitrile (100 mL) is added 4-fluorobenzenethiol (2.95 g, 23.00 mmol) and potassium carbonate (4. H g, 29.78 mmol). The mixture is heated at reflux for 18 hours. The mixture is diluted with water and extracted with ethyl acetate. Organic phase is evaporated in vacuo and purified by flash chromatography (5-100% ethyl acetate in hexanes) to give the title compound (6.00 g, 100 %).
References:
WO2007/106705,2007,A1 Location in patent:Page/Page column 86

109384-19-2
469 suppliers
$5.00/1g

226398-48-7
11 suppliers
inquiry