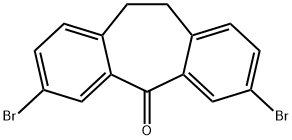
3,7-Dibromo-10,11-dihydro-dibenzo[a,d]cyclohepten-5-one synthesis
- Product Name:3,7-Dibromo-10,11-dihydro-dibenzo[a,d]cyclohepten-5-one
- CAS Number:226946-20-9
- Molecular formula:C15H10Br2O
- Molecular Weight:366.05
![3,7-diamino-10,11-dihydro-5H-dibenzo[a,d][7]annulen-5-one](/CAS/20200611/GIF/96886-97-4.gif)
96886-97-4
0 suppliers
inquiry
![3,7-Dibromo-10,11-dihydro-dibenzo[a,d]cyclohepten-5-one](/CAS/20180703/GIF/226946-20-9.gif)
226946-20-9
7 suppliers
inquiry
Yield:226946-20-9 70%
Reaction Conditions:
with tert.-butylnitrite;copper(ll) bromide in acetonitrile; for 2 h;Inert atmosphere;Reflux;
Steps:
3,7-dibromodibenzosuberone, 6
To a solution of 3,20 g (14.3mmol) of CuBr2 and 1.7 ml (14.3mmol) tert-butylnitrite in freshly distilled, dry MeCN under nitrogen, a suspension of 1.7 g (7.1mmol) of 5 in dry MeCN was slowly added. The resulting solution was stirred over night at room temperature and then heated to reflux for another 2 h. After cooling to room temperature, 30 ml of a solution of 10% HCl in H2O was slowly added. The organic layer was washed with a solution of 10% HCl in H2O; the aqueous layer was washed several times with Et2O. The combined organic layers were dried over MgSO4 and purified by flash chromatography (light petroleum ether). After evaporating the solvent 1.80 g of the product were obtained as a white solid (70%). 1H NMR (300.1 MHz, acetone-d6): δ: 8.13 (d, 2H, 4JHH = 2.20 Hz, H1), 7.56 (dd, 2H, 3JHH = 7.96 Hz, 4JHH = 2.20 Hz, H2), 7.12 (d, 2H, 3JHH = 7.96 Hz, H3), 3.14 (s, 4H, H4).
References:
Mücke, Philipp;Zabel, Manfred;Edge, Ruth;Collison, David;Clément, Sébastien;Záli?, Stanislav;Winter, Rainer F. [Journal of Organometallic Chemistry,2011,vol. 696,# 20,p. 3186 - 3197] Location in patent:supporting information; experimental part

1210-35-1
297 suppliers
$6.00/1g
![3,7-Dibromo-10,11-dihydro-dibenzo[a,d]cyclohepten-5-one](/CAS/20180703/GIF/226946-20-9.gif)
226946-20-9
7 suppliers
inquiry
![5H-Dibenzo[a,d]cyclohepten-5-one, 10,11-dihydro-3,7-dinitro-](/CAS/20210305/GIF/96886-98-5.gif)
96886-98-5
0 suppliers
inquiry
![3,7-Dibromo-10,11-dihydro-dibenzo[a,d]cyclohepten-5-one](/CAS/20180703/GIF/226946-20-9.gif)
226946-20-9
7 suppliers
inquiry