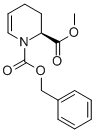
METHYL (2S)-1-CBZ-1,2,3,4-TETRAHYDRO-2-PYRIDINECARBOXYLATE synthesis
- Product Name:METHYL (2S)-1-CBZ-1,2,3,4-TETRAHYDRO-2-PYRIDINECARBOXYLATE
- CAS Number:227758-97-6
- Molecular formula:C15H17NO4
- Molecular Weight:275.3
![L-Norleucine, 6-hydroxy-N-[(phenylmethoxy)carbonyl]-, methyl ester](/CAS/20200611/GIF/154061-64-0.gif)
154061-64-0
0 suppliers
inquiry

227758-97-6
7 suppliers
inquiry
Yield:227758-97-6 62.3%
Reaction Conditions:
Stage #1: (2S)-2-<(Benzyloxycarbonyl)amino>-6-hydroxyhexanoic acid methyl esterwith sulfur trioxide pyridine complex;triethylamine in dimethyl sulfoxide at 30 - 40; for 2.5 h;
Stage #2: with toluene-4-sulfonic acid in N,N-dimethyl-formamide;toluene; for 2 h;Reflux;
Steps:
8.8
The ZME (2.92 g) obtained in Example 7 was placed in a solution of dimethylsulfoxide (hereinafter referred to as "DMSO") (5 mL) containing triethylamine (6.2 g). To the resulting solution, a solution of pyridine sulfur trioxide complex (4.19 g) in DMSO (13 mL) was further added dropwise at 30 to 40 ° C. over 2.5 hours. After completion of the reaction, methylene chloride (13 mL) and water (18 mL) were added and the layers were separated. The aqueous layer was extracted with methylene chloride (13 mL). The methylene chloride solutions used for the extraction were combined, washed three times with a 10% aqueous citric acid solution (40 mL) and once with water (5 mL), dried over MgSO 4 and concentrated under reduced pressure to obtain an oxidation product It was. The obtained oxidation product was dissolved in toluene (30.8 mL) and dimethylformamide (hereinafter referred to as "DMF") (407 μL), and paratoluenesulfonic acid monohydrate (200 mg) was added thereto And the mixture was heated under reflux for 2 hours. Ethyl acetate (31 mL) was added to the obtained reaction solution, and the mixture was washed with aqueous sodium bicarbonate solution (31 mL). The organic layer was dried over sodium sulfate Na 2 SO 4 (12.3 g), filtered, and concentrated under reduced pressure. The concentrated residue thus obtained was purified with a silica gel column (elution solvent: toluene → toluene: ethyl acetate = 19: 1 → toluene: ethyl acetate = 9: 1) to obtain DZME as a colorless oil. The yield of this example was 1.695 g, and the yield was 62.3%.
References:
JP2015/40200,2015,A Location in patent:Paragraph 0268; 0269; 0270
![1,3-Dioxolane-2-pentanoic acid, α-[[(phenylmethoxy)carbonyl]amino]-, methyl ester, (αS)-](/CAS/20210305/GIF/824943-43-3.gif)
824943-43-3
0 suppliers
inquiry

227758-97-6
7 suppliers
inquiry
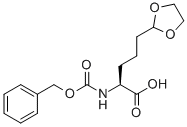
852822-01-6
7 suppliers
inquiry

227758-97-6
7 suppliers
inquiry
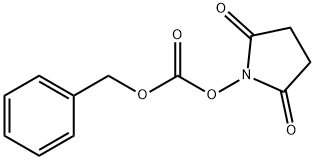
13139-17-8
503 suppliers
$6.00/25g

227758-97-6
7 suppliers
inquiry
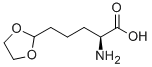
215054-80-1
23 suppliers
$201.00/250mg

227758-97-6
7 suppliers
inquiry