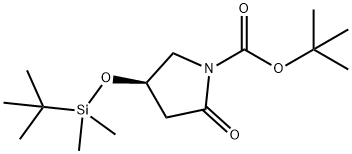
4-(tert-Butyl-dimethyl-silanyloxy)-2-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-(tert-Butyl-dimethyl-silanyloxy)-2-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester
- CAS Number:228267-20-7
- Molecular formula:C15H29NO4Si
- Molecular Weight:315.48
Yield:228267-20-7 95%
Reaction Conditions:
with dmap;triethylamine in acetonitrile at 0 - 20;
Steps:
6.B Step B:
(R)-tert-butyl 4-(tert-butyldimethylsilyloxy)-2-oxopyrrolidine-1-carboxylate
To a solution of (R)-4-(tert-butyldimethylsilyloxy)pyrrolidin-2-one (9.64 g, 44.8 mmol) in CH3CN (90 mL) were added TEA (7.49 mL, 53.7 mmol), DMAP (5.47 g, 44.8 mmol) and (t-Boc)2O (12.5 mL, 53.7 mmol) at 0° C.
The reaction mixture was stirred at room temperature overnight and then partitioned between EtOAc and water.
The separated organic layer was washed with saturated aq. NH4Cl and brine, dried over Na2SO4, filtered and concentrated in vacuo.
The residue was purified by column chromatography on SiO2 (Hex:EtOAc=3:1) to afford (R)-tert-butyl 4-(tert-butyldimethylsilyloxy)-2-oxopyrrolidine-1-carboxylate (13.4 g, 95%) as a pale brown solid. 1H-NMR (CDCl3, Varian 400 MHz): δ 0.077 (3H, s), 0.082 (3H, s), 0.88 (9H, s), 1.53 (9H, s), 2.47 and 2.71 (2H, ABq, JAB=17.4 Hz), 3.62 and 3.86 (2H, ABq, JAB=11.3 Hz), 4.37-4.41 (1H, m).
References:
US2016/168156,2016,A1 Location in patent:Paragraph 0217; 0221; 0222
18162-48-6
665 suppliers
$9.00/5g

228267-20-7
2 suppliers
inquiry

![(4R)-4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-pyrrolidinone](/CAS/20180703/GIF/131653-51-5.gif)