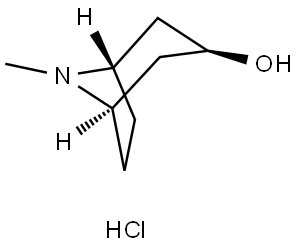
8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl-, hydrochloride (1:1), (3-endo)- synthesis
- Product Name:8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl-, hydrochloride (1:1), (3-endo)-
- CAS Number:2292-08-2
- Molecular formula:C8H15NO.ClH
- Molecular Weight:177.67
120-29-6
401 suppliers
$10.00/5g
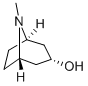
![8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl-, hydrochloride (1:1), (3-endo)-](/CAS/20240320/GIF/2292-08-2.gif)
2292-08-2
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in diethyl ether;
Steps:
8-Methyl-8-azabicyclo[3.2.1]octan-3a-ol was converted into the corresponding hydrochloride by treatment with dry HCl gas, in ethyl ether. Then, 2 g (1.13 mmol) of 8-methyl-8-azabicyclo[3.2.1]octan-3a-ol hydrochloride (1) was suspended in 25 ml of dry acetonitrile and 4.2 ml (34.8 mmol) of trichloromethyl chloroformate were added dropwise at 0 °C. The reaction mixture was stirred at this temperature for 30 min and then at room temperature for 24 h more, until a clear solution was obtained. The solvent was removed under vacuum and the residue triturated with ethyl ether to afford 2 pure enough to be used in the following step.
References:
Iriepa;Bellanato [Journal of Molecular Structure,2011,vol. 1006,# 1-3,p. 508 - 512] Location in patent:experimental part