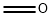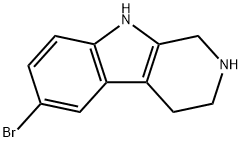
1H-PYRIDO[3,4-B]INDOLE, 6-BROMO-2,3,4,9-TETRAHYDRO- synthesis
- Product Name:1H-PYRIDO[3,4-B]INDOLE, 6-BROMO-2,3,4,9-TETRAHYDRO-
- CAS Number:23046-69-7
- Molecular formula:C11H11BrN2
- Molecular Weight:251.12
Yield:23046-69-7 38.39%
Reaction Conditions:
Stage #1: formaldehyd;5-bromotryptamine in methanol;toluene; for 24 h;Heating / reflux;
Stage #2: with potassium hydroxide in water; pH=12;
Steps:
69
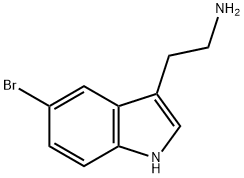
To a stirred solution of 2-(5-bromo-lH-indol-3-yl)ethanamine (1.Og, 3.64 mmol) in methanol: toluene (1:1, 20 mL) was added paraformaldehyde (200 mg, 20%). The reaction mass was refiuxed for 24h. The progress of the reaction was monitored by TLC and after completion, the reaction mass was concentrated and the crude was washed with ethyl acetate. The aqueous layer was adjusted to pH=12 with 20% KOH solution. The precipitated solid was extracted with ethyl acetate and the organic layer washed with brine solution and concentrated to give the tetrahydrocarboline (350 mg, 38.39%). 1HNMR (200 MHz, DMSOd6) δ: 2.65 (t, 2H, CH2), 3.0 (t, 2H, CH2), 3.92 (s, 2H, CH2), 7.1 (d, IH, Ar- H), 7.21 (d, IH, Ar-H), 7.45 (s, IH, Ar-H), 10.95 (s, IH, NH); m/e = 251 (M+l).To a stirred solution of the tetrahydrocarboline (350 mg, 1.4 mmol) in DMF (20 mL) were added Example 7 (453 mg, 2.09 mmol) and potassium carbonate (580 mg, 4.20 mmol) and the mixture was heated to 80-90° C for 2-3h. The progress of the reaction was followed by TLC and after completion DMF was distilled off under vacuum. The residue was partitioned between ethyl acetate (150 mL) and water and the two layers were separated. The aqueous layer was extracted with ethyl acetate (150 mL) and both the organic layers were combined. The ethyl acetate layer was dried over anhydrous sodium sulfate, concentrated under vacuum and the residue was purified by washing with diethyl ether (100 mL) to give Example 69 (250 mg, 46.31%). 1HNMR (200 MHz, DMSO-d6) δ: 2.93 (t, 2H, CH2), 3.8 (s, 3H, OCH3), 4.28 (t, 2H, CH2), 5.0 (s, 2H, CH2), 7.18 (d, IH, Ar-H), 7.22 (d, IH, Ar-H), 7.7 (s, IH, Ar-H), 8.82 (s, 2H, Pyrimidine-H), 11.2 (s, IH N-H); m/e = 387(M+1).
References:
WO2006/88949,2006,A1 Location in patent:Page/Page column 131; 132
![1,2,3,4-TETRAHYDRO-9H-PYRIDO[3,4-B]INDOLE](/CAS/GIF/16502-01-5.gif)
16502-01-5
152 suppliers
$8.00/100mg
![1H-PYRIDO[3,4-B]INDOLE, 6-BROMO-2,3,4,9-TETRAHYDRO-](/CAS/GIF/23046-69-7.gif)
23046-69-7
9 suppliers
inquiry
145489-90-3
6 suppliers
inquiry
![1H-PYRIDO[3,4-B]INDOLE, 6-BROMO-2,3,4,9-TETRAHYDRO-](/CAS/GIF/23046-69-7.gif)
23046-69-7
9 suppliers
inquiry
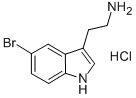
81868-12-4
78 suppliers
$75.09/250mg

298-12-4
444 suppliers
$5.00/25g
![1H-PYRIDO[3,4-B]INDOLE, 6-BROMO-2,3,4,9-TETRAHYDRO-](/CAS/GIF/23046-69-7.gif)
23046-69-7
9 suppliers
inquiry