
5-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:5-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID METHYL ESTER
- CAS Number:23170-45-8
- Molecular formula:C6H8N2O2
- Molecular Weight:140.14

67-56-1
760 suppliers
$7.29/5ml-f
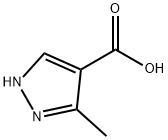
40704-11-8
110 suppliers
$9.00/250mg

23170-45-8
71 suppliers
$53.00/100mg
Yield: 57%
Reaction Conditions:
with thionyl chloride at 0; for 12 h;
Steps:
5 Synthesis of methyl 3-rnetIiy1-1H-pyraioie-4-carboxyIate.
Synthesis of /V-((2-hydroxy-4,( methyl-l-(l-benzrl)-l /-pyrazoie-4-carboxamide (Compound 11 esis 14] To a solution of 3-methyi-lH-pyrazole-4-carboxylic acid (1.26 g, 10 mmol) in methanol (100 mL) was added thionyl chloride (5.73 g, 45 mmol) at 0 °C. The mixture was stirred for 12 hours. The solvent was evaporated in vacuo. To the residue, saturated sodium bicarbonate aqueous solution was added and the mixture was extracted with ethyl acetate (100 mLx 3), the organic phase was dried by sodium, sulfate. The mixture was filtered and the filtrate was concentrated in vacuo to give methyl 3-m.ethyl-lH~pyrazole~4~carboxylate (0.8 g, 57%). H NMR (300 MHz, CDCI3): δ 7.87 (s, 1H), 3.84 (s, 3H), 2.53 (s, 3H).
References:
CONSTELLATION PHARMACEUTICALS;ALBRECHT, Brian, K.;AUDIA, James, Edmund;COOK, Andrew, S.;DAKIN, Les, A.;DUPLESSIS, Martin;GEHLING, Victor, S.;HARMANGE, Jean-Christophe;NAVESCHUK, Christopher, G.;VASWANI, Rishi, G. WO2013/120104, 2013, A2 Location in patent:Paragraph 00183; 00184
4637-24-5
616 suppliers
$5.00/25g
105-45-3
668 suppliers
$5.00/5g

23170-45-8
71 suppliers
$53.00/100mg
23326-27-4
144 suppliers
$38.13/1g
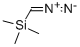
18107-18-1
211 suppliers
$33.00/5g

68809-58-5
45 suppliers
$144.00/50mg

23170-45-8
71 suppliers
$53.00/100mg

105-45-3
668 suppliers
$5.00/5g

23170-45-8
71 suppliers
$53.00/100mg