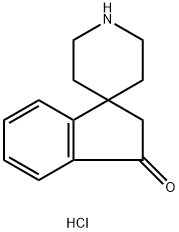
spiro[indene-1,4'-piperidin]-3(2H)-one hydrochloride synthesis
- Product Name:spiro[indene-1,4'-piperidin]-3(2H)-one hydrochloride
- CAS Number:231938-20-8
- Molecular formula:C13H16ClNO
- Molecular Weight:237.73
![N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE](/CAS/GIF/159634-59-0.gif)
159634-59-0
85 suppliers
$60.00/25mg
![spiro[indene-1,4'-piperidin]-3(2H)-one hydrochloride](/CAS/20150408/GIF/231938-20-8.gif)
231938-20-8
18 suppliers
$144.00/50mg
Yield:231938-20-8 97.6%
Reaction Conditions:
with hydrogenchloride;methanol
Steps:
15
The mixture of N-Boc protected spiroindanone 15a (20 g, 66.4 mmol) and MeOH/HCl (2.5 mol/L, 100 mL) were stirred overnight. After evaporation the residue was washed by petroleum ether to gave the corresponding amine hydrochloride 15b (15.4 g, 97.6%). [0264] To a solution of compound 15b (5.0 g, 24.84 mmol) and Et3N (7.54 g, 74.53 mol) in CH2C12 (50 mL) was added drop-wise Cbz-Cl (4.66 g, 27.33 mmol) at 0 °C. The reaction was allowed to warm to room temperature and stirred overnight. The precipitate was filtered, washed with Et20 and dried to furnish compound 15c (6.1 g, yield 99%). [0265] A solution of compound 15c (3 g, 10.3 mmol) in EtOH (30 mL) containing NH2OH.HCl (1.43 g, 20.6 mmol) and NaOAc (1.52 g, 18.53 mmol) was heated under reflux for 1.5 h. The solvent was removed by evaporation and the residue was partitioned between CH2CI2 and water. The organic phase was washed with brine, dried over Na2SC>4, and concentrated to give compound 15d (3.14 g, yield 99%), which could be used directly in the next step. [0266] 2,4,6-trichloro-[l,3,5]-triazine (1.32 g, 7.16 mmol) was added to DMF (9.6 mL) maintained at 25 °C. The reaction was monitored by TLC until TCT was consumed. Then compound 15d (1.6 g, 4.77 mmol) in DMF (17 mL) was added. After the addition, the mixture was stirred at room temperature overnight. Water was added. The mixture was extracted with EtOAc. The combined organic layers were washed with sat. Na2CC>3, followed by IN HC1 and brine, dried over Na2S04 and concentrated. The residue was purified by prep HPLC to obtain compound 15e (260 mg, yield 16%).[0267] The mixture of compound 15e (1.2 g, 3.4 mmol) and Pd/C (200 mg) in MeOH (20 mL) was hydrogenated under atmosphere pressure at room temperature for 3 h. The catalyst was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by preparative HPLC twice to give 15f (110 mg, 11 %) as a TFA salt. *H NMR (CDCI3) 8 7.65 (d, .7=7.5 Hz,l H), 7.29-7.45 (m, 3 H), 3.45 (d, J= 12.3 Hz, 2 H), 3.20 (t, J= 12.3 Hz, 2 H), 2.96 (s, 2 H), 2.10-2.21 (m, 2 H), 1.70 (d, J= 14.1 Hz, 2 H). MS (ESI) m/z 211.06 [M+H]+. [0268] Amine 15f (22 mg, 0.1 mmol) and ethyl 4-formylpiperidine-l-carboxylate (28 mg, 0.15 mmol) were dissolved in DCE (1 mL) and NaBH(OAc)3 (42 mg; 0.2 mmol) was added. The reaction was stirred at room temperature for 16 h. The reaction was diluted methanol (0.5 mL), filtered, and compound no. 34 was purified using reversed-phase chromatography (10-99% CH3CN/H2O gradient with 0.05% TFA). LC/MS m/z 386.2 [M+H]+, retention time 2.05 min (10-99% CH3CN-H2O gradient with 0.03% TFA, 5 min); .H NMR (free base, 400 MHz, DMSO-d6) 5 7.71 (d, J= 3.9 Hz, 2H), 7.61 (d, J= 7.6 Hz, 1H), 7.47-7.41 (m, 1H), 4.05-3.96 (m, 4H), 2.86-2.67 (m, 4H), 2.56 (d, J= 9.7 Hz, 2H), 2.18 (s, 2H), 2.01 (d, J= 7.7 Hz, 4H), 1.73 (d, .7=11.1 Hz, 4H), 1.45 (d, J= 8.7 Hz, 2H), 1.18 (t, /= 7.1 Hz, 3H), 1.03-0.96 (m, 2H).
References:
WO2006/23852,2006,A2 Location in patent:Page/Page column 105; 106