
5,7-BIS-(BENZYLOXY)-2-(4-(BENZYLOXY)PHENYL)-3-HYDROXY-4H-CHROMEN-4-ONE synthesis
- Product Name:5,7-BIS-(BENZYLOXY)-2-(4-(BENZYLOXY)PHENYL)-3-HYDROXY-4H-CHROMEN-4-ONE
- CAS Number:23405-70-1
- Molecular formula:C36H28O6
- Molecular Weight:556.6

96333-59-4
17 suppliers
$85.00/5mg

23405-70-1
14 suppliers
$109.90/5mg
Yield:23405-70-1 78%
Reaction Conditions:
Stage #1: 5,7-bis(benzyloxy)-2-(4-(benzyloxy)phenyl)-4H-chromen-4-onewith 3,3-dimethyldioxirane in dichloromethane;acetone at 0;
Stage #2: toluene-4-sulfonic acid in dichloromethane at 0; for 0.5 h;
Steps:
To a solution containing LOO g (1.85 mmol) of 3 in 30 mL of CH2Cl2 at 00C was added 30 mL of a 0.9-0.11 M solution of DMDO in acetone. The reaction mixture was stirred at 0 0C overnight under N2. The solvent was concentrated under diminished pressure, and then the residue was redissolved in 50 mL OfCH2Cl2 and treated with catalytic pTsOH. The reaction mixture was stirred at 0 0C for 30 min. The solvent was concentrated under diminished pressure and the residue was purified by flash chromatography on a silica gel column (25 x 4 cm). Elution with 1:1 hexanes-ethyl acetate gave 4 as a light brown solid: yield 0.63 g (78% based on consumed starting material) and 0.22 g of unreacted starting material; silica gel TLC Rf 0.28 (3:1 hexanes-ethyl acetate); 1H NMR (DMSO-J6) δ 5.19 (s, 2H), 5.25 (s, 2H), 6.69 (s, IH), 6.97 (br s, IH), 7.18 (d, 2H, J= 8.5 Hz), 7.30-7.44 (m, 11 H), 7.46-7.50 (m, 2H), 7.67 (d, 2H, J= 7.5 Hz), 8.14 (d, 2H, J= 8.5 Hz) and 9.15 (s, IH); 13C NMR (DMSO-^6) δ 70.1, 70.5, 70.7, 94.6, 98.0, 102.2, 107.4, 115.6, 127.2, 128.1, 128.5, 128.6, 128.7, 128.87, 128.92, 129.16, 129.23, 129.4, 136.8, 137.4, 137.6, 138.8, 142.5, 158.7, 159.5, 159.8, 163.4 and 171.9; mass spectrum (FAB), m/z 551.1961 (M + H)+ (C36H29O6 requires 557.1964).
References:
WO2006/86103,2006,A2 Location in patent:Page/Page column 4-5; 25; 34
![2-Propen-1-one, 1-[2-hydroxy-4,6-bis(phenylmethoxy)phenyl]-3-[4-(phenylmethoxy)phenyl]-](/CAS/20210305/GIF/23243-67-6.gif)
23243-67-6
0 suppliers
inquiry

23405-70-1
14 suppliers
$109.90/5mg
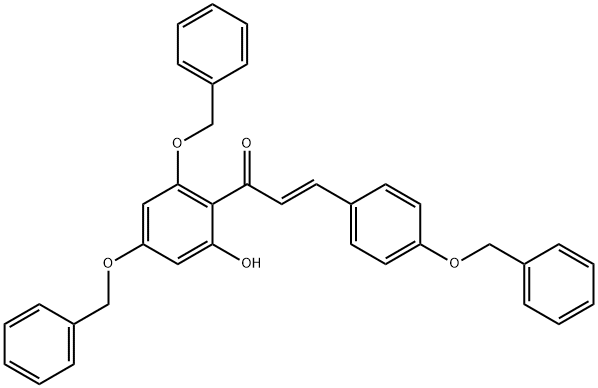
88607-79-8
16 suppliers
inquiry

23405-70-1
14 suppliers
$109.90/5mg
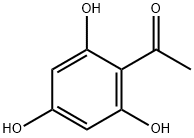
480-66-0
345 suppliers
$10.00/1g

23405-70-1
14 suppliers
$109.90/5mg
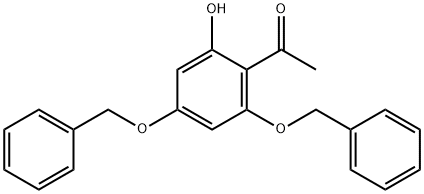
18065-05-9
50 suppliers
$42.00/250mg

23405-70-1
14 suppliers
$109.90/5mg