
1,2-BenzenediaMine, 4-[6-(4-Methyl-1-piperazinyl)-1H-benziMidazol-2-yl]- synthesis
- Product Name:1,2-BenzenediaMine, 4-[6-(4-Methyl-1-piperazinyl)-1H-benziMidazol-2-yl]-
- CAS Number:23491-49-8
- Molecular formula:C18H22N6
- Molecular Weight:322.41
![4-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-2-nitroaniline](/CAS/20180629/GIF/23623-05-4.gif)
23623-05-4
7 suppliers
inquiry
![1,2-BenzenediaMine, 4-[6-(4-Methyl-1-piperazinyl)-1H-benziMidazol-2-yl]-](/CAS/20150408/GIF/23491-49-8.gif)
23491-49-8
3 suppliers
inquiry
Yield:23491-49-8 98%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in methanol;ethyl acetate at 20; under 760.051 Torr;Product distribution / selectivity;
Steps:
6
Reference Example 6 - Preparation of 2-amino-4-[5'-(4"-methylpiperazin-1'-yl)benzimidazol-2'-yl]aniline (Reference Compound 6); The title compound was prepared as detailed by Kelly et al. Physical and NMR data have been previously detailed by Kelly et al. A solution of 4-[5'-(4"-methylpiperazin-1"-yl)benzimidazol-2'-yl]-2-nitroaniline (560 mg, 1.32 mmol) and palladium on carbon (5%, 115 mg) in methanol/ethyl acetate (20:80, 40 mL) was hydrogenated under 1 atmosphere of hydrogen at room temperature. When the uptake of hydrogen (approximately 96 mL) had ceased the solution was filtered through celite and the solvent removed to give the product as a orange solid (510 mg, 98 %).
References:
EP2044938,2009,A2 Location in patent:Page/Page column 20
![4-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-2-nitroaniline](/CAS/20180629/GIF/23623-05-4.gif)
23623-05-4
7 suppliers
inquiry
![1,2-BenzenediaMine, 4-[6-(4-Methyl-1-piperazinyl)-1H-benziMidazol-2-yl]-](/CAS/20150408/GIF/23491-49-8.gif)
23491-49-8
3 suppliers
inquiry
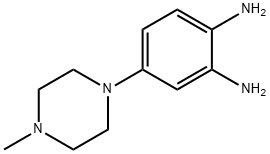
54998-08-2
89 suppliers
$87.00/250mg
![1,2-BenzenediaMine, 4-[6-(4-Methyl-1-piperazinyl)-1H-benziMidazol-2-yl]-](/CAS/20150408/GIF/23491-49-8.gif)
23491-49-8
3 suppliers
inquiry
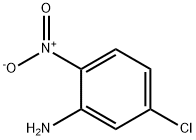
1635-61-6
293 suppliers
$13.00/25g
![1,2-BenzenediaMine, 4-[6-(4-Methyl-1-piperazinyl)-1H-benziMidazol-2-yl]-](/CAS/20150408/GIF/23491-49-8.gif)
23491-49-8
3 suppliers
inquiry

109-01-3
651 suppliers
$5.00/5G
![1,2-BenzenediaMine, 4-[6-(4-Methyl-1-piperazinyl)-1H-benziMidazol-2-yl]-](/CAS/20150408/GIF/23491-49-8.gif)
23491-49-8
3 suppliers
inquiry