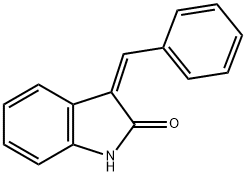
(3Z)-3-(phenylmethylidene)-2,3-dihydro-1H-indol-2-one synthesis
- Product Name:(3Z)-3-(phenylmethylidene)-2,3-dihydro-1H-indol-2-one
- CAS Number:23772-61-4
- Molecular formula:C15H11NO
- Molecular Weight:221.25
Yield:23772-61-4 92.8%
Reaction Conditions:
with sodium hydroxide for 2 h;Cooling with ice;Claisen-Schmidt Condensation;
Steps:
General procedure for the synthesis of compounds (3-20)
General procedure: The target oxindole derivatives (chalcones) (3-20) were prepared by continuous stirring of oxindole (0.1 mole, 1.33 g) (1) and appropriate aldehyde (0.1 mol) (2) in an alcoholic solution (80%, 25 ml) containing 2% sodium hydroxide, in ice-cold conditions for about 2 h (Scheme 1). The mixtures of chalcones obtained were refrigerated for about 10 h (completion of the reactions were monitored by TLC) and were neutralized by pouring into 20%, 200 ml HCl with continuous stirring. The solids precipitated out were filtered off, washed with water and recrystallized from ethanol to give final purified compounds (3-20).
References:
Suthar, Sharad Kumar;Bansal, Sumit;Alam, Md. Maqusood;Jaiswal, Varun;Tiwari, Amit;Chaudhary, Anil;Alex, Angel Treasa;Joseph, Alex [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 22,p. 5281 - 5285] Location in patent:supporting information
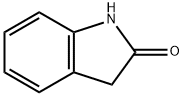
59-48-3
462 suppliers
$5.00/1g

100-52-7
945 suppliers
$21.30/2g

23772-61-4
6 suppliers
inquiry

23782-37-8
1 suppliers
inquiry
201230-82-2
1 suppliers
inquiry
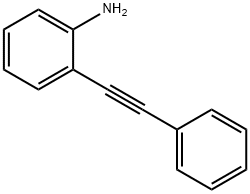
13141-38-3
38 suppliers
$20.00/100mg
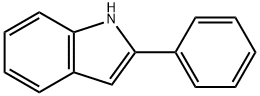
948-65-2
287 suppliers
$5.00/10g

23772-61-4
6 suppliers
inquiry

23782-37-8
1 suppliers
inquiry

23782-37-8
1 suppliers
inquiry

23772-61-4
6 suppliers
inquiry
