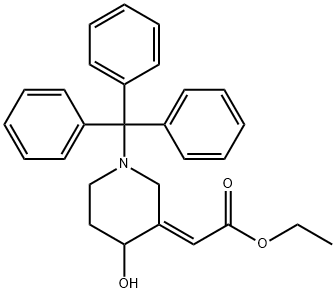
rac- (2E)-3-[(Ethoxycarbonyl)Methylene]-1-trityl-4-piperidinol synthesis
- Product Name:rac- (2E)-3-[(Ethoxycarbonyl)Methylene]-1-trityl-4-piperidinol
- CAS Number:239466-40-1
- Molecular formula:C28H29NO3
- Molecular Weight:427.53
![(2E)-2-[4-Oxo-1-trityl-3-piperidinylidene]acetic Acid Ethyl Ester](/CAS/GIF/239466-39-8.gif)
239466-39-8
9 suppliers
$498.80/5MG
![rac- (2E)-3-[(Ethoxycarbonyl)Methylene]-1-trityl-4-piperidinol](/CAS/GIF/239466-40-1.gif)
239466-40-1
7 suppliers
inquiry
Yield:239466-40-1 57.9%
Reaction Conditions:
with methanol;sodium tetrahydroborate in dichloromethane at 20; for 1 h;Cooling with ice;
Steps:
3 Synthesis of ethyl-(E)-2-(4-hydroxy-1-tritylpiperidin-3-enyl)acetate
After dissolving 22g (0.0517mol) ethyl-(E)-2-(4-carbonyl-1-tritylpiperidin-3-enyl)acetate in 50mL dichloromethane, add 200mL methanol, and under ice-cold conditions After adding 2.34 g (0.0619 mol) of sodium borohydride little by little, the mixture was stirred at room temperature for 1 h.After the reaction solution was concentrated under reduced pressure, 150 mL of water was added, extracted with ethyl acetate, and the organic layer was dried over anhydrous sodium sulfate. The solvent was concentrated under reduced pressure, and the obtained residue was purified by silica gel column chromatography to obtain light yellow oil M3-7, 12.8 g, with a yield of 57.9%.
References:
CN111362864,2020,A Location in patent:Paragraph 0085-0088

76-83-5
688 suppliers
$6.00/25g
![rac- (2E)-3-[(Ethoxycarbonyl)Methylene]-1-trityl-4-piperidinol](/CAS/GIF/239466-40-1.gif)
239466-40-1
7 suppliers
inquiry

112257-60-0
44 suppliers
$59.00/1g
![rac- (2E)-3-[(Ethoxycarbonyl)Methylene]-1-trityl-4-piperidinol](/CAS/GIF/239466-40-1.gif)
239466-40-1
7 suppliers
inquiry