
2,7-DIMETHYLPYRIDO[1,6-A]-1H-IMIDAZOLE-3-CARBOXYLIC ACID, ETHYL ESTER synthesis
- Product Name:2,7-DIMETHYLPYRIDO[1,6-A]-1H-IMIDAZOLE-3-CARBOXYLIC ACID, ETHYL ESTER
- CAS Number:241146-66-7
- Molecular formula:C12H14N2O2
- Molecular Weight:218.25
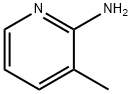
1603-40-3
480 suppliers
$6.00/25g
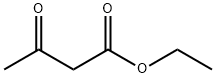
141-97-9
780 suppliers
$5.00/25g
![2,7-DIMETHYLPYRIDO[1,6-A]-1H-IMIDAZOLE-3-CARBOXYLIC ACID, ETHYL ESTER](/StructureFile/ChemBookStructure2/GIF/CB0443049.gif)
241146-66-7
23 suppliers
$174.00/1g
Yield:241146-66-7 92 %
Reaction Conditions:
with oxone;potassium iodide in ethanol;water at 20;Sonication;
Steps:
General procedure for synthesisof imidazo[1,2-a]-pyridine-3-carboxylates (IPCs)
General procedure: KI-Oxone (1.0 equiv) was slowly added to a well-stirredsolution of β-ketoester (1 mmol) and 2-aminopyridines (1 mmol) in ethanol: H2O(3 ml), and the reaction mixturewas allowed to go under ultrasonic irradiation at room temperaturefor 22-30 min. On completion of the reaction, asindicated by TLC (ethyl acetate: ether, 25:75), the solventwas evaporated under reduced pressure. Then obtained solidwas dissolved in sodium hydrogen carbonate saturated solutionand extracted three times with chloroform. The combinedorganic extracts were washed with water, dried overanhydrous Na2SO4,and concentrated under a vacuum toafford the products. The crude products were purified bysilica gel (60-120 mesh; 5.5 g) column chromatographyusing hexane/ethyl acetate (9:1 v/v) as eluent.
References:
Desai, Vikram;Patil, Sandip;Nipane, Sandip;Sawant, Vijay;Kurane, Rajanikant;Deshmukh, Madhukar;Patil, Suresh [Journal of the Iranian Chemical Society,2023]

1603-40-3
480 suppliers
$6.00/25g
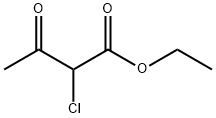
609-15-4
445 suppliers
$6.00/25g
![2,7-DIMETHYLPYRIDO[1,6-A]-1H-IMIDAZOLE-3-CARBOXYLIC ACID, ETHYL ESTER](/StructureFile/ChemBookStructure2/GIF/CB0443049.gif)
241146-66-7
23 suppliers
$174.00/1g