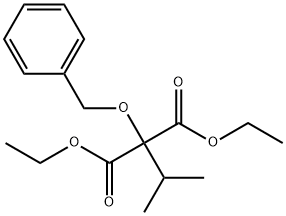
2-ISOPROPYL-2-(BENZYLOXY)-PROPANEDIOIC ACID 1,3-DIETHYL ESTER synthesis
- Product Name:2-ISOPROPYL-2-(BENZYLOXY)-PROPANEDIOIC ACID 1,3-DIETHYL ESTER
- CAS Number:24124-03-6
- Molecular formula:C17H24O5
- Molecular Weight:308.37
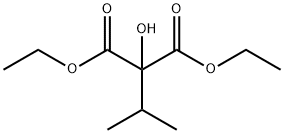
24124-04-7
15 suppliers
$640.00/250mg

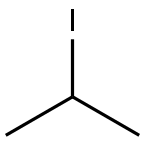
100-39-0
431 suppliers
$10.00/10g

24124-03-6
13 suppliers
$95.00/50mg
Yield:24124-03-6 80.3%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-d6-formamide;mineral oil at 70 - 75; for 2 h;Cooling with ice;
Steps:
Synthesis of compound diethyl 2-(benzyloxy)-2-isopropylmalonate (7)
To a suspension of NaH (0.8 g, 0.033 mol, 60% oildispersion) in anhydrous N,N-dimethylformamide(12.5 mL), then diester 6 (3.4 g, 0.016 mmol) wasadded dropwise under ice-bath. When no gas wasgenerated during the reaction, the reaction mixturewas heated to 70 °C, and benzyl bromide (3.20 g,0.019 mol) was added dropwise. After that, the mixturewas stirred at 75 °C for 2 h. Then ethanol(2.00 mL) was added and stirred at 75 °C for another30 min to quench the excess benzyl bromide. Thereaction mixture was neutralized with acetic acidafter cooling to room temperature, diluted withwater (150 mL), then extracted with petroleum ether(3 × 70 mL), the organic phase was washed successivelywith saturated sodium carbonate and water,dried over anhydrous sodium sulfate, filtrated, andsolvent was removed under reduced pressure. Thecrude residue was purified on a silica gel column toafford the pure 7 as a yellow oil 3.6 g, 80.3% yield andthe purity was 98% by gas chromatography. 1H NMR(400 MHz, CDCl3): δ 7.40 (2H, d, J = 7.3 Hz),7.36-7.23 (3H, m), 4.75 (2H, s), 4.26 (4H, q, J= 7.1 Hz), 2.56-2.49 (1H, m), 1.29 (6H, t, J= 7.1 Hz), 1.05, (6H, d, J = 6.9 Hz).
References:
Ding, Jing;Yang, Zhijun;Zhao, Yu;Fang, Weizhen;Lu, Qun [Bioscience, Biotechnology and Biochemistry,2019,vol. 83,# 4,p. 763 - 767]

24182-44-3
2 suppliers
inquiry

24124-03-6
13 suppliers
$95.00/50mg
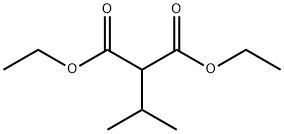
759-36-4
175 suppliers
$17.00/10g

24124-03-6
13 suppliers
$95.00/50mg