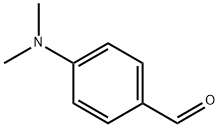
4-(Cyclopenta-2,4-dien-1-ylidenemethyl)-N,N-dimethylaniline synthesis
- Product Name:4-(Cyclopenta-2,4-dien-1-ylidenemethyl)-N,N-dimethylaniline
- CAS Number:2428-22-0
- Molecular formula:C14H15N
- Molecular Weight:197.28
Yield:2428-22-0 98%
Reaction Conditions:
with pyrrolidine in methanol;water;Inert atmosphere;
Steps:
4.4. Synthesis of (2-ylidene)cyclopenta-1,3-dienes 3. Method C. General procedure
General procedure: To a solution of aldehyde or ketone (5 mmol) and cyclopentadiene (0.3966 g, 6.0 mmol/0.492 mL for the crystalline fulvenes or 0.4 mL/5 mmol for the aliphatic substrates) in MeOH/H2O (5 mL 4/1) pyrrolidine (0.0355 g, 10 mol %) was added. The formed crystals were filtered, washed with small amounts of cold MeOH/H2O mixture and dried in air. For the isolation of oily products the reaction mixture was transferred to an ice-cold mixture of brine solution and 10 mol % AcOH in a narrow graduated cylinder. The organic phase pure was pipetted out. Drying over molecular sieves produced practically pure (>95%) fulvene.
References:
Cokun, Necdet;Erden, Ihsan [Tetrahedron,2011,vol. 67,# 45,p. 8607 - 8614] Location in patent:experimental part