
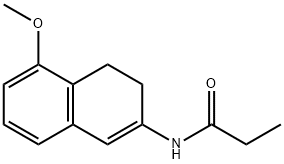
N-[(2S)-1,2,3,4-Tetrahydro-5-methoxy-2-naphthalenyl]propanamide synthesis
- Product Name:N-[(2S)-1,2,3,4-Tetrahydro-5-methoxy-2-naphthalenyl]propanamide
- CAS Number:244239-67-6
- Molecular formula:C14H19NO2
- Molecular Weight:233.31
1321942-91-9
9 suppliers
inquiry
![N-[(2S)-1,2,3,4-Tetrahydro-5-methoxy-2-naphthalenyl]propanamide](/CAS/20180703/GIF/244239-67-6.gif)
244239-67-6
14 suppliers
inquiry
Yield: 28%
Reaction Conditions:
with hydrogen at 30; under 18751.9 Torr; for 18 h;Inert atmosphere;
Steps:
Synthesis of (S)-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)propionamide(10).
The reaction was performed in a 50 mL Parr pressure vessel fitted with a glassliner, stirrer bar, injection port, bursting disk, pressure relief valve and pressure gauge. The glass liner was charged with substrate 9 (1.0 g, 4.32 mmol) and [RuCl((R)-T-BINAP)2(µ-Cl)3][NH2Me2](15 mg, 8.64.10-3 mmol), the vessel was sealed and flushed fivetimes with nitrogen (25 bar). MeOH (10 mL, anhydrous and deoxygenated) was added to the vessel via the injection port and the system was flushed three times with hydrogen (25 bar). The vessel was heated to 30 °C, and stirred (1,000 rpm) for 18 h. The stirring was reduced to 500 rpm and the vessel allowed to cool to room temperature, vented to atmosphere, flushed once with nitrogen (25 bar) and opened. An aliquot of the reaction mixture was analysed by SFC and 1H NMR spectroscopy. This showed the reaction had gone to completion with an ee of 91% (>99% yield). The reaction mixture was reduced to dryness and recrystallised from hot ethylacetate to yield white crystals of >98% ee (28%). 1H NMR (CDCl3): d 1.15 (3H,CH3, t, J3 8.0Hz), 1.74-1.83 (1H, CHHCHN, m), 1.98-2.06 (1H, CHHCHN, m), 2.18 (2H, CH2CO,q, J3 8.0 Hz), 2.63 (1H, CHHCHN,dd, J2 16.0 Hz, J3 8.0 Hz), 2.68-2.82 (2H, CH2, m), 3.10 (1H, CHHCHN, dd, J2 20.0 Hz, J3 8.0 Hz), 3.82 (3H, OCH3,s), 4.26-4.34 (1H, CHN, m), 5.47 (1H, NH, br d), 6.69 (2H, Ar, d,J3 8.0 Hz), 7.10(1H, Ar, t, J3 8.0 Hz). SFC method (for e.e.determination of 10). Conditions: OD-H column, 250x4.6 mm x 5 µm, MeOH/CO2 (90:10): 4.15 min (R enantiomer), 5.13 min (enamide), 6.04min (S enantiomer).
References:
Cobley, Christopher J.;Evans, George;Fanjul, Tamara;Simmonds, Shaun;Woods, Amy [Tetrahedron Letters,2016,vol. 57,# 9,p. 986 - 989] Location in patent:supporting information
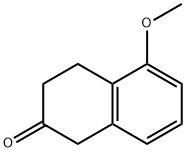
32940-15-1
316 suppliers
$13.43/250mgs:
![N-[(2R)-1,2,3,4-Tetrahydro-5-methoxy-2-naphthalenyl]propanamide](/CAS/20180529/GIF/244239-68-7.gif)
244239-68-7
6 suppliers
inquiry
![N-[(2S)-1,2,3,4-Tetrahydro-5-methoxy-2-naphthalenyl]propanamide](/CAS/20180703/GIF/244239-67-6.gif)
244239-67-6
14 suppliers
inquiry

32940-15-1
316 suppliers
$13.43/250mgs:
![N-[(2S)-1,2,3,4-Tetrahydro-5-methoxy-2-naphthalenyl]propanamide](/CAS/20180703/GIF/244239-67-6.gif)
244239-67-6
14 suppliers
inquiry