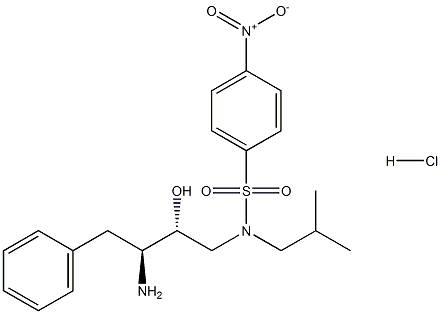
N-((2R,3S)-3-AMINO-2-HYDROXY-4-PHENYLBUTYL)-N-ISOBUTYL-4-NITROBENZENE-1-SULFONAMIDE HYDROCHLORIDE synthesis
- Product Name:N-((2R,3S)-3-AMINO-2-HYDROXY-4-PHENYLBUTYL)-N-ISOBUTYL-4-NITROBENZENE-1-SULFONAMIDE HYDROCHLORIDE
- CAS Number:244634-31-9
- Molecular formula:C20H28ClN3O5S
- Molecular Weight:457.97
![tert-Butyl [(1S,2R)-1-benzyl-2-hydroxy-3-[isobutyl[(4-nitrophenyl)sulfonyl]amino]propyl]carbamate](/CAS/GIF/191226-98-9.gif)
191226-98-9
95 suppliers
inquiry

244634-31-9
14 suppliers
inquiry
Yield:244634-31-9 330 g
Reaction Conditions:
with hydrogenchloride in ethanol;dichloromethane;water at 5 - 80; for 2 h;Solvent;
Steps:
1.c Preparation of (1S,2R)-3-[[4-(Nitrophenyl)sulfonyl](2-methylpropylamino)]-2-hydroxy-1-(phenyl methyl)propyl]-amine as its hydrochloride salt. (Amino nitro alcohol hydrochloride)
Methylene chloride solution of step (b) was concentrated at 30-45° C. under reduced pressure. To the concentrated mass, ethanol (2375 ml) and hydrochloric acid (35% w/w, 170 ml) was added and heated at 75-80° C. for 1 h. Thereafter the reaction mixture was cooled to 5-10° C. and stirred at this temperature for 1 h. The product obtained was filtered, washed with ethanol (440 ml) and dried at 45-55° C. under reduced pressure to obtain amino nitro alcohol hydrochloride. [0090] Yield: 330 g [0091] Chromatographic Purity (by HPLC): 99.92%
References:
US2015/141382,2015,A1 Location in patent:Paragraph 0089; 0090; 0091
![tert-Butyl [(1S,2R)-1-Benzyl-2-hydroxy-3-(isobutylamino)propyl]carbamate](/CAS/GIF/160232-08-6.gif)
160232-08-6
107 suppliers
$26.00/100mg

244634-31-9
14 suppliers
inquiry